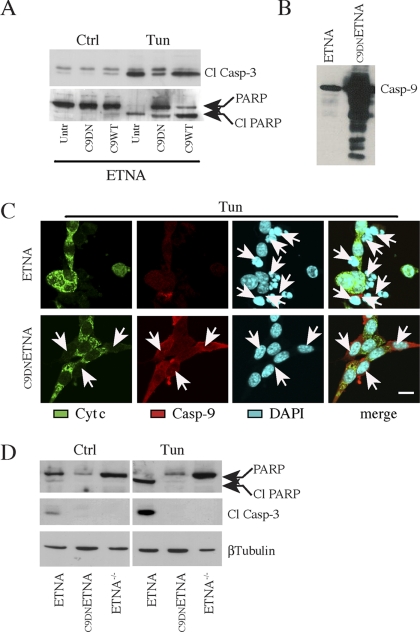
Figure 1.
ETNA cells overexpressing a dominant negative form of caspase-9 (C9DNETNA) are resistant to apoptotic stimuli. (A) Total cell lysates from untransfected ETNA cells (Untr), ETNA cells transiently transfected with dominant negative caspase-9 (C9DN) or ETNA cells transiently transfected with wild-type caspase-9 (C9WT) untreated (Ctrl) or treated for 40 h with 3 μg/ml tunicamycin (Tun) were subjected to SDS-PAGE and analyzed for caspase-3 processing and for PARP processing. (B) Western blot analysis of caspase-9 expression in untransfected ETNA cells (ETNA) or ETNA cells stably overexpressing caspase-9 (C9DNETNA). (C) Triple-labeling immunofluorescence microscopy of ETNA and C9DNETNA cells treated for 40 h with 3 μg/ml tunicamycin (Tun). Cytochrome c (green), caspase-9 (red), nuclear staining (DAPI, blue), and merge of the three patterns are shown. ETNA cells show release of cytochrome c and extensive nuclear fragmentation (white arrows), whereas C9DNETNA cells exhibit released cytochrome c but unaffected nuclei (white arrows). Notice that in the immunofluorescence assay the used anti-caspase-9 antibody (which can recognize both the procaspase-9 and its cleaved form) enables caspase-9 detection only upon its overexpression (in C9DNETNA cells). Scale bar, 20 μm. (D) Western blot analysis of caspase-3 processing and PARP processing in total lysates of ETNA, C9DNETNA, and ETNA−/− cells untreated (Ctrl) or treated with tunicamycin (Tun) as described before. Immunoblot detection of β-tubulin is used as control of equal protein loading.