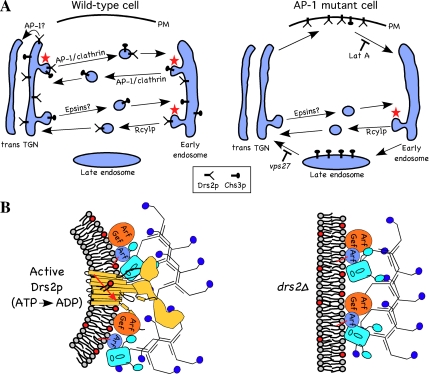
Figure 8.
Models for the codependency of Drs2p and AP-1 in vesicle-mediated protein transport. (A) AP-1 and clathrin are required for anterograde transport of Drs2p from the TGN to the early endosome. Alternatively, it is possible that AP-1 mediates retrieval of Drs2 from the TGN to earlier Golgi cisternae (pathway marked AP-1?). Anterograde transport of Chs3p occurs, for the most part, independently of AP-1 and perhaps requires the Golgi epsins (Ent3p and Ent5p). AP-1 mutant cells treated with Lat A accumulate all of Drs2p and a fraction of Chs3p on the plasma membrane. Retrograde transport of Drs2p from the early endosome back to the TGN does not depend on AP-1, but appears to use the Rcy1p pathway. In contrast, Chs3p depends solely on AP-1 for early endosome to TGN retrograde transport. AP-1 mutants missort most of Chs3p, but no Drs2p, to the late endosome where it can be trapped behind a vps27 block. Pathways that require Drs2p activity for vesicle formation are indicated with a red star. (B) In the absence of Drs2p (drs2Δ), AP-1 and clathrin are recruited to the membrane, but vesicles are not produced because the coat components cannot induce sufficient curvature in the membrane. (C) Enzymatically active Drs2p (yellow), modeled with a similar structure as SERCA1(Toyoshima et al., 2000), is required for budding AP-1/clathrin-coated vesicles. Interaction of Drs2p with Arf guanine nucleotide exchange factors (Arf Gef; Chantalat et al., 2004) and AP-1 should concentrate activated Arf-GTP, AP-1 and clathrin at sites of lipid translocation where the clathrin coat can efficiently mold the curved membrane into a vesicle.