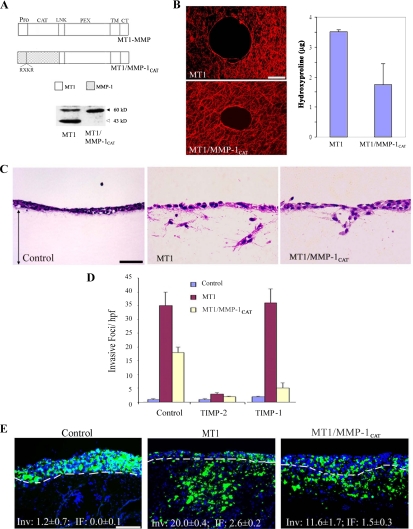
Figure 3.
A tethered collagenase mimics MT1-MMP activity. (A) Schematic diagram of MT1-MMP and the MT1/MMP-1CAT chimera display the locations of the pro-, catalytic (CAT), linker (LNK), hemopexin (PEX), transmembrane (TM) and cytoplasmic (CT) domains of the respective proteinases. The furin-recognition sequence inserted in the MMP-1 predomain is shown for the chimeric protein and depicted as RXKR (Arg-X-Lys-Arg). Western blot analysis of cell surface expression of MT1-MMP and MT1/MMP-1CAT was assessed after surface biotinylation and immunoprecipitation (the level of MT1/MMP-1CAT is 55% of total wt MT1-MMP as quantified by ImageQuant 5.2). The active and autodegraded forms of wild-type MT1-MMP migrate as ∼60- and ∼43-kDa bands (marked with black and gray arrows, respectively). The MT1/MMP-1CAT appears only as a ∼56-kDa band in a manner consistent with the inability of the MMP-1 catalytic domain to cleave within the MT1-MMP hinge region (Osenkowski et al., 2005). (B) Pericellular collagenolysis of MT1-MMP- and MT1/MMP-1CAT–expressing COS cells as assessed by confocal laser microscopy (bar, 50 μm) and hydroxyproline release, respectively. Results for collagen degradation are expressed as the mean micrograms of hydroxyproline ± SEM of three experiments. (C and D) Type I collagen gel invasion by control-, MT1-MMP-, or MT1/MMP-1CAT–transfected COS cells was assessed in the absence or presence of either TIMP-1 (2 μg/ml) or TIMP-2 (2 μg/ml). Representative cross sections of H&E-stained gels are shown in C (bar, 50 μm), with invasive activity quantified in D. Results are expressed as the mean ± SEM of three or more experiments. (E) Fluorescent micrographs of CAM cross sections after culture with control-, MT1-MMP- or MT1/MMP-1CAT–transfected COS cells for 3 d. Results are representative of three or more experiments performed. Bar, 100 μm.