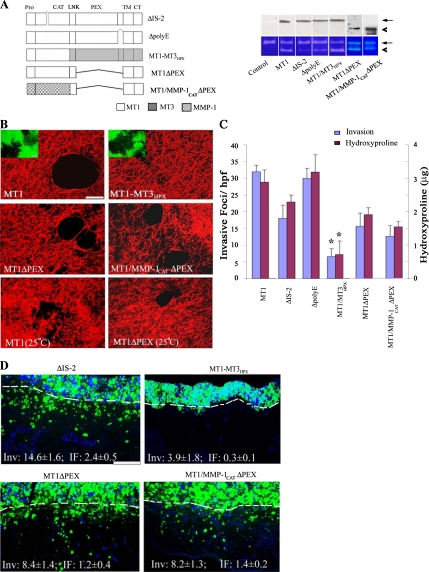
Figure 4.
Domain-dependent regulation of MT1-MMP–mediated collagenolysis and invasive activities. (A) Schematic diagram of MT1-MMP mutants, ΔIS-2, ΔpolyE, MT1-MMP3HPX, MT1ΔPEX, and MT1/MMP-1CATΔPEX. Deleted domains are marked by the symbol “⋀,” whereas MT1-MMP, MT3-MMP, and MMP-1 domains are shaded white, gray, and patterned, respectively. COS cells were transfected with control-, MT1-MMP-, ΔIS-2-, ΔpolyE, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX expression vectors. Expression levels of membrane-localized MT1-MMP or MT1-MMP variants were determined by Western blot analysis after surface biotinylation (top; arrow and arrowhead mark position of active forms of the proteinase). The cell surface expression levels of MT1-MMP variants were 109, 113, 133, 103, and 167% of MT1-MMP, respectively. The processing of exogenous MMP-2 by the transfected cells was monitored by gelatin zymography (bottom). The pro- and active forms of MMP-2 are marked by arrow and arrowhead, respectively. (B) Laser confocal micrographs of pericellular collagenolytic (bar, 50 μm) or gelatinolytic (insets; bar, 50 μm) activity expressed by COS cells transfected with control, MT1-MMP, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX expression vectors and cultured at 37°C. In the bottom two panels, the pericellular collagenolytic activity expressed by COS cells transfected with MT1-MMP or MT1ΔPEX cultured at 25°C is confirmed. (C) Invasion and collagenolytic activities were quantified as invasive foci/hpf (mean ± SEM of 5 randomly selected fields in a single representative experiment of 5 performed) and as micrograms of hydroxyproline released as described (mean ± SEM; n = 3). *p < 0.1 versus MT1. (D) Fluorescent micrographs of CAM cross sections after a 3-d culture with ΔIS-2-, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX transfectants. The control values for MT1-MMP–transfected COS cells are Inv, 20.0 ± 0.4 and IF, 2.6 ± 0.2 (results representative of 3 or more experiments; see Figure 3E). Results shown are representative of three or more experiments performed. Bar, 100 μm.