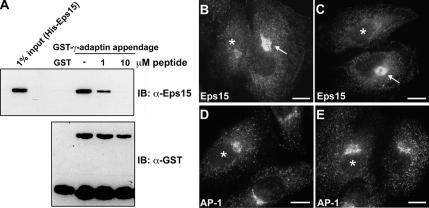
Figure 4.
Disruption of the Eps15–AP-1 interaction reduces recruitment of Eps15 to the trans-Golgi network. The association between Eps15 and AP-1 is reduced by the competitive action of a 14-amino acid peptide representing the C-terminal residues of the DPF repeats domain both in vitro and in vivo. (A) GST or GST-tagged γ-adaptin appendage domain was immobilized on glutathione-Sepharose beads and incubated with full-length His-Eps15 in the absence or presence (1 or 10 μM) of the 14-amino acid Eps15 peptide (720ESFGDGFADFSTLS733). The pulldowns were immunoblotted with anti-Eps15 and anti-GST antibodies. The input lane represents 1% of total His-Eps15 used for the pulldown. The interaction between the γ-adaptin appendage domain and Eps15 was significantly reduced as the peptide concentration increased. (B–E) To test whether the Eps15 peptide might also reduce the Eps15–AP-1 interaction within the confines of living cells, HeLa cells were microinjected with 100 nM peptide then allowed to recover for 6 h before immunostaining for Eps15 and AP-1. Peptide-injected cells (*) showed a dramatic reduction in Eps15 staining (B and C) in the Golgi region, compared with uninjected cells (arrows) or cells injected with buffer alone (not shown). In contrast, the Golgi localization pattern of AP-1 was not altered by peptide injection (D and E). Scale bars, 20 μm.