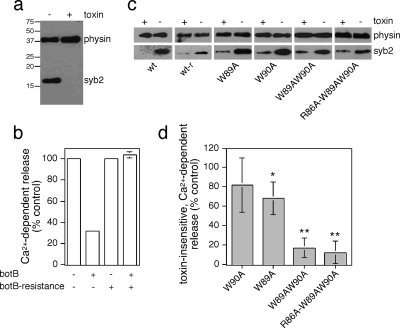
Figure 5.
Toxin-insensitive, mutant syb2 is unable to rescue secretion from PC12 cells. (a) PC12 cells were permeabilized with 10 μM digitonin and incubated in the presence or absence of botB/LC, followed by detection of intact syb2 coil by Western blotting. Blots were reprobed for synaptophysin (physin) to determine equal amounts of protein loading. (b) PC12 cells, transfected with a plasmid encoding for hGH as well as wild-type or botulinum toxin-resistant syb2 (Q76V,F77W) were permeabilized with 10 μM digitonin and incubated in the presence or absence of botB/LC. Ca2+-dependent hGH release was evoked by 10 μM Ca2+ for 10 min and compared with basal release (0 μM Ca2+). The amount of hGH in the medium and in the cells was determined by an enzyme-linked immunosorbent assay, and the percentage of secreted hGH, and the total amount of hGH, were calculated against an hGH standard curve. The graph is a representative of two independent experiments with duplicate data points. Error bars are only shown if larger than bar columns. (c) Cells were transfected with wild-type or toxin-resistant wild-type syb2 (wt-r), or toxin-resistant mutant syb2 as indicated, and treated with botB/HnLC upon permeabilization to compare expression levels. (d) To standardize results from repeated experiments, secretion observed in the presence of toxin-insensitive, wild-type syb2 was set to 100%, and the relative lack of rescue of secretion of test plasmids in the presence of toxin normalized to this control. Values are means ± SEM (n = 3–5). The statistical significance of differences from wild-type were analyzed by a Student's t test (*p < 0.05; **p < 0.01).