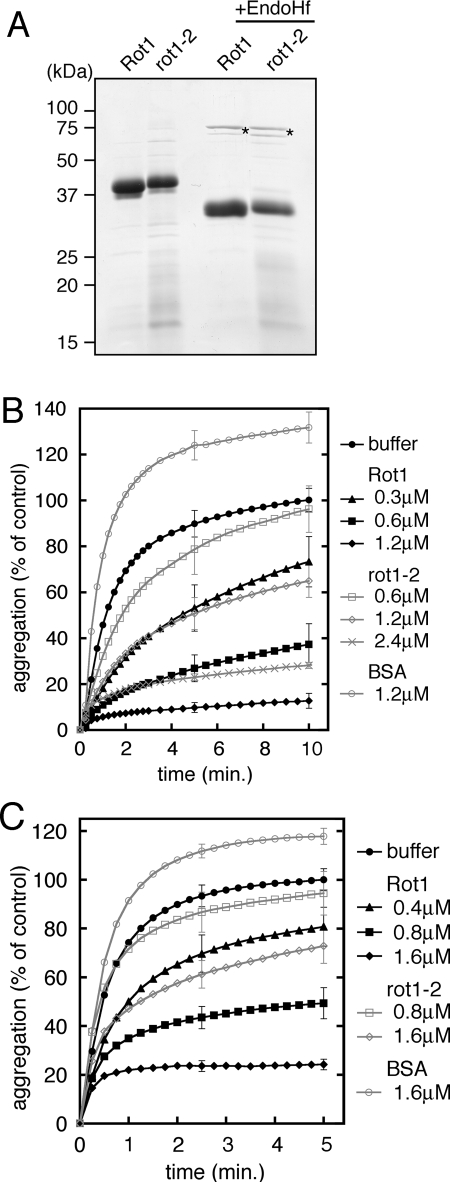
Figure 1.
Recombinant Rot1 prevents aggregation of denatured proteins in vitro. (A) Purified recombinant Rot1. Wild-type Rot1 and rot1-2 proteins were expressed and purified as described in Materials and Methods. Protein, 3 μg each, was resolved by SDS-PAGE (12%), and the gel was stained with Coomasie Brilliant Blue. Where indicated, the proteins were treated with EndoHf (New England Biolabs, Beverly, MA) according to manufacturer's instructions. Asterisks, EndoHf. (B and C) Rot1 prevents aggregation of denatured α-mannosidase and citrate synthase. α-Mannosidase (B) or citrate synthase (C) was denatured with 4 M guanidine-HCl and diluted 50-fold to 0.3 μM (B) or 0.4 μM (C) at time 0. Indicated amounts of Rot1, rot1-2, or control protein BSA was incubated together, and A320 was measured at RT for the indicated periods. A320 of the buffer alone reaction at 10 min (B) or 5 min (C) is set to 100%, and the relative extent of the aggregation of denatured α-mannosidase or citrate synthase for each conditions of the reaction is shown. The averages of at least three reactions are shown. For simplicity, SDs are shown only for 5 and 10 min (B) or 2.5 and 5 min (C). Because the purity of rot1-2 was lower than Rot1, the actual concentration of rot1-2 in the reactions was estimated to be ∼75% that of Rot1.