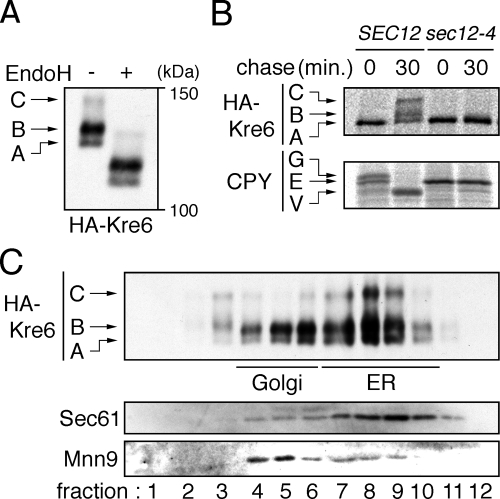
Figure 3.
Modification and localization of Kre6. (A) Western blot detection of HA-Kre6. ROT1 cells expressing HA-Kre6 (YM41) was incubated in YPD at 30°C, and the cell lysates were subjected to anti-HA Western blotting. Where indicated, the lysate was treated with EndoHf. The positions of the three forms of HA-Kre6 not treated with EndoHf are indicated in the left of the panel. (B) Conversion of HA-Kre6 requires transport from the ER to Golgi. The cells (YM41 and YM132 [ROT1 HA-KRE6 sec12-4]) were incubated in SC for 1 h at 23°C, labeled with [35S]Met/Cys for 10 min at 33°C and chased for 30 min at 33°C. The cells were lysed, and HA-Kre6 or CPY was immunoprecipitated, separated by SDS-PAGE and detected by autoradiography. The positions of each forms of HA-Kre6 and CPY (E, ER; G, Golgi; V, vacuole) are indicated. (C) Intracellular distribution of HA-Kre6. YM41 was incubated in YPD at 30°C, spheroplasted and lysed gently with a Dounce homogenizer. The lysate was fractionated by a 20–80% sucrose step gradient ultracentrifugation. The fractions were subjected to Western blotting to detect HA-Kre6, Mnn9, and Sec61. Mnn9 and Sec61 were used as a Golgi or an ER marker, respectively.