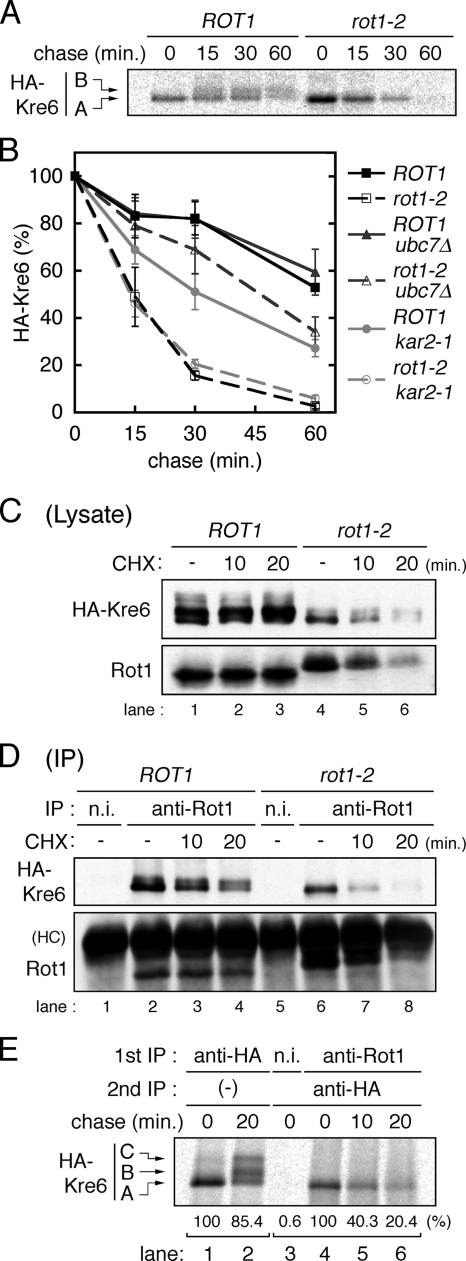
Figure 4.
Rot1 is a molecular chaperone for Kre6. (A) Conversion of HA-Kre6 does not occur in the rot1-2 mutant. ROT1 and rot1-2 cells expressing HA-Kre6 (YM41 and YM42) were incubated for 10 min at 37°C, labeled with [35S]Met/Cys for 10 min and chased for indicated periods at 37°C. Radiolabeled HA-Kre6 was recovered and detected as in Figure 3B. The positions of the A and the B forms of Kre6 are indicated in the left of the panel. The C form was barely detected under the conditions of this experiment. (B) HA-Kre6 is degraded by the ERAD in the rot1-2 cells. Pulse-chase experiments for HA-Kre6 were performed as in A, using the HA-KRE6 strains: YM41, YM42, and YM43 (ROT1 ubc7Δ); YM44 (rot1-2 ubc7Δ); YM90 (ROT1 kar2-1); and YM91 (rot1-2 kar2-1). Radioactive signals of HA-Kre6 (the A plus the B forms) were quantified, and the averages and SDs of at least five independent experiments are shown. Signal intensities of HA-Kre6 at the beginning of the chase period are set to 100%. (C and D) Rot1 interacts with HA-Kre6. Cells (YM41 and YM42) were grown in YPD at 23°C and incubated at 37°C for 10 min. Where indicated, the cells were further incubated with CHX (0.1 mg/ml) for the indicated times. Cell lysis and IP using anti-Rot1 antibody or a nonimmune serum (n.i.; as a negative control) were performed under a nondenaturing conditions at 4°C. The cell lysates (C) or the immunoprecipitants (D) were analyzed by Western blotting using anti-HA and anti-Rot1 antibodies. HC, immunoglobulin heavy chain. (E) Transient interaction of Rot1 and Kre6. Cells (YM41) were incubated, labeled with [35S]Met/Cys and chased for the indicated periods as in A. Anti-HA IP (lanes 1 and 2) or double-IP against Rot1 (or nonimmune serum) first and then HA (lanes 3–6) were performed. Relative amounts of recovered radioactive HA-Kre6 in the representative experiments are shown under the panel.