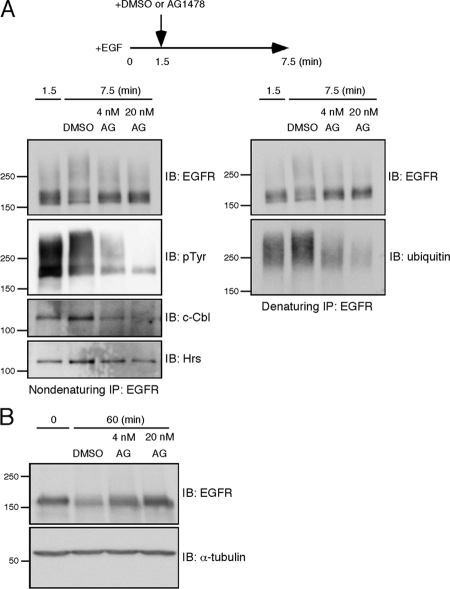
Figure 7.
Inhibition of tyrosine phosphorylation leads to rapid deubiquitination of EGFR. (A) HeLa cells were stimulated with EGF for 1.5 min, and then AG1478 was added to the medium at a final concentration of 4 or 20 nM. As a control, an equal volume of DMSO was added. The cells were further incubated for 6 min. EGFR was subject to two rounds of immunoprecipitation. Tyrosine-phosphorylated EGFR, c-Cbl, and Hrs were detected from the first, nondenaturing immunoprecipitates. Ubiquitin-conjugated EGFR was detected from the second, denaturing ones. (B) HeLa cells were treated as described in A. After the addition of DMSO or AG1478, the cells were further incubated for 58.5 min to monitor EGFR degradation. As a control, cells were lysed before EGF stimulation. EGFR was detected by the immunoblotting of the whole cell lysates.