Figure 5.
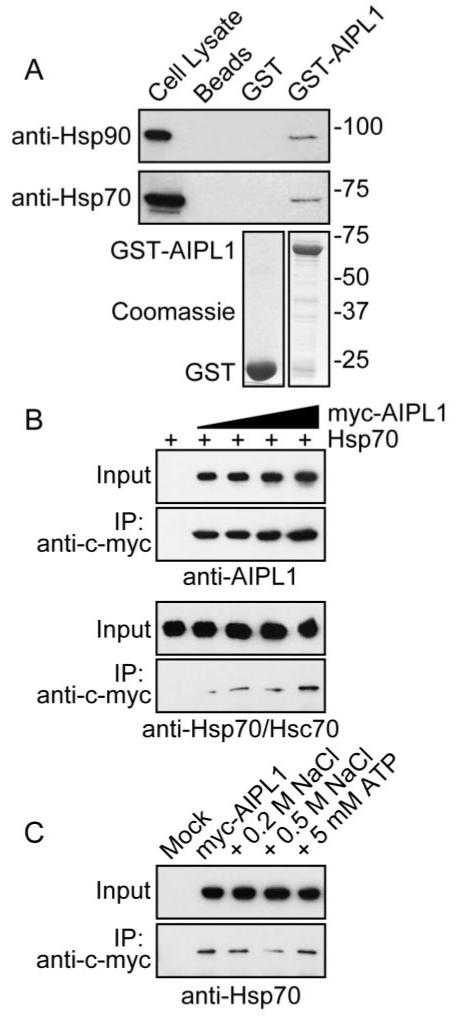
Characterization of AIPL1-chaperone interactions using in vitro protein-binding assays. (A) Affinity pull-down of endogenous Hsp90 and Hsp70 with GST-AIPL1. GST and GST-AIPL1 were affinity purified on glutathione Sepharose 4B (lower; Coomassie stain). COS7 cell lysates were applied to GST (GST) or GST-AIPL1 (GST-AIPL1) prebound to glutathione Sepharose 4B. Endogenous Hsp90 and Hsp70 pulled down with GST-AIPL1 were detected with anti-Hsp90 or anti-Hsp70 (upper; Western blot). Molecular weight markers are in kDa. (B) Coimmunoprecipitation of Hsp70 with myc-AIPL1. Myc-AIPL1 was immunoprecipitated from transfected SK-N-SH lysates with anti-c-myc 9E10. Myc-AIPL1 and Hsp70 were detected in the input and immune complex with anti-AIPL1 and anti-Hsp70/Hsc70, respectively. (C) Coimmunoprecipitation of Hsp70 with myc-AIPL1 from SK-N-SH cells was examined in the presence of increasing ionic strength or ATP/Mg2+.
