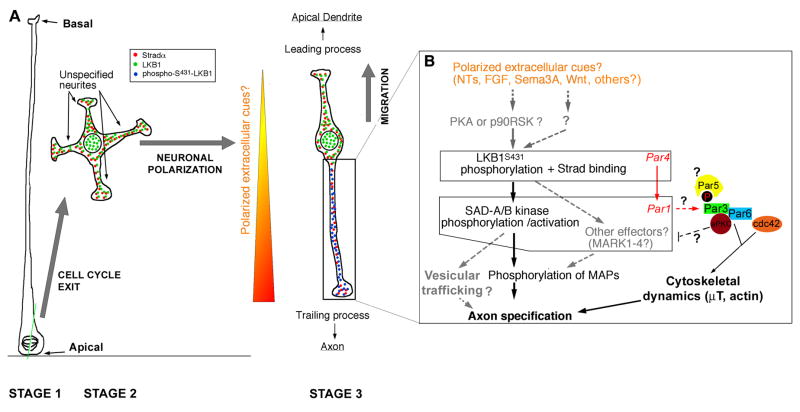
Figure 3. Molecular mechanisms underlying LKB1 function in neuronal polarization in vivo.
(A) Upon asymmetric cell division of radial glial progenitors (Stage 1), early unpolarized post-mitotic neurons show a transient phase of non-directed neurite outgrowth in the subventricular zone (Stage 2) before adopting a bipolar morphology in the intermediate zone where they engage radial migration with a leading process directed towards the pial surface and a trailing process directed towards the ventricle. (B) Based on recent reports [21,55], we propose that in vivo, the trailing process is specified to become the axon in response to putative extracellular cues that preferentially induce phosphorylation of LKB1 on Serine431. This event might be mediated in part by cues providing chemotactic attraction of radially migrating neurons towards the cortical plate such as Sema3A [70] or any other extracellular cues neurotrophins (NTs) such as BDNF/NT4/NT3 [55], Wnt [66,67], FGFs or other cues that can activate cAMP-dependent protein kinase (PKA) or p90 RSK (RSK1–3). One cannot exclude the possibility of another uncharacterized serine/threonine protein kinase can phosphorylate Serine 431 in vivo and play a role in neuronal polarization. Once LKB1 is activated by binding to its necessary co-activator Strad (αor β) and S431-phosphorylation (which occurs only in the neurite becoming the axon), LKB1 phosphorylates SAD-A/B kinases (and likely Microtubule Affinity-Regulated Kinases, MARK1–4) which are required for axon specification in part by phosphorylating microtubule-associated proteins such as Tau. Based on the function of SAD-kinases in presynaptic vesicular clustering in C. elegans [77], we can hypothesize that SAD-A/B kinases might also specify axon identity by directing vesicular trafficking in the neurite becoming the axon. Based on evidence obtained in D. melanogaster, Par1 can phosphorylate Par3 on two serine residues that constitute binding sites for the 14-3-3 protein Par5, an event that controls its localization during D. melanogaster oocyte polarity. At present this is the only potential link between the Par3/Par6/aPKC complex and Par4/Par1 dyad during cell polarization. See text for details. Modified from [21].