Figure 1.
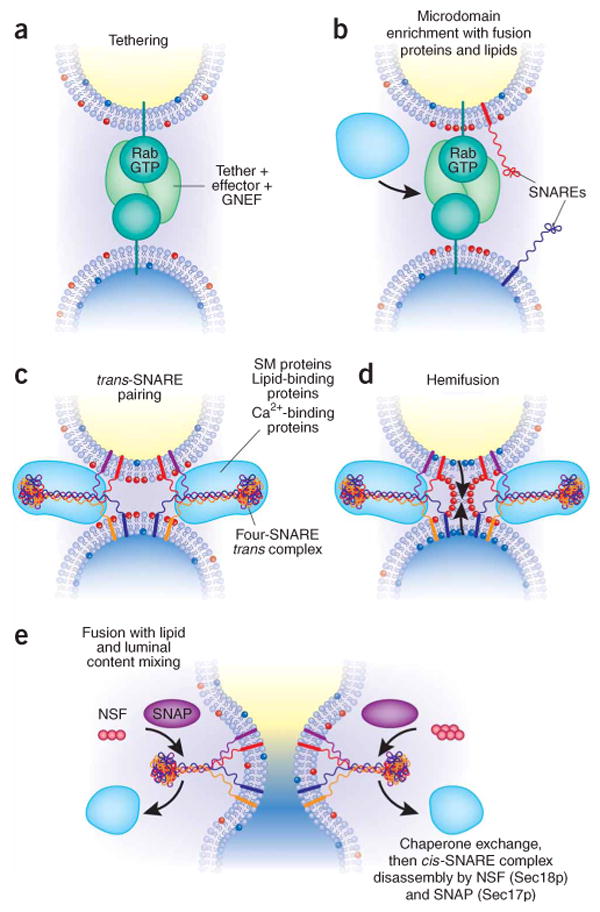
Membrane fusion on the exocytic and endocytic pathways, in five steps. (a) The first association of membranes, termed ‘tethering’, requires a prenyl-anchored Rab-family GTPase and tethering proteins termed ‘effectors’124, which bind to the Rab in its activated, GTP-bound state. Proteins mediating tethering have been studied in the Golgi stacks125,126 and other systems. (b) Rab-regulated enrichment of fusion proteins and lipids in a microdomain. Rabs, their multi-functional effector complexes, and lipids with defined roles in fusion (such as sterols (not shown) or phosphoinositides or diacylglycerol (red polar head groups)) assemble into a microdomain, the site of subsequent steps in the fusion pathway. In some systems, Rab effectors can include guanine nucleotide exchange factors, which activate Rabs; lipid kinases, which synthesize phosphoinositides; and SM proteins, which bind SNAREs. It remains unclear in most instances whether Rab effectors must remain bound to the Rab to be activated for these functions, or whether concentration of these several protein and lipid factors in the fusion microdomain suffices. In some systems, such as the yeast vacuole, one multisubunit complex fulfills tethering, guanine nucleotide exchange, SM protein, and lipid-binding functions. (c) Assembly of trans-SNARE complexes127 with additional regulatory proteins. These include SM proteins48 and can include proteins or domains that bind to Ca2+, to lipids or to SNAREs. These complexes may encircle the site of future fusion44. Lipids with small head groups and negative membrane curvature, which promote hemifusion, are enriched at the cytoplasmic surface of the fusion microdomain (red head groups). (d) Hemifusion, formed by fusion of the halves of the lipid bilayer of each membrane that face the cytoplasm. Arrows indicate the direction of subsequent lipid movement to complete the fusion process. Lipids with positive curvature due to large head groups (shown here as blue head groups) may become enriched at this stage for invasion of the hemifused structure (arrows). (e) Completion of fusion, with mixing of lipid bilayers, membrane proteins and luminal compartments but retention of the barrier between cytoplasm and organellar lumen. This process converts trans-SNARE complexes to post-fusion cis-SNARE complexes; it is unclear whether cis-SNARE complexes can arise by any other route. SNAP (Sec17p) may displace other SNARE-bound proteins and prepare the cis-SNARE complex for ATP-dependent disassembly by NSF (Sec18p).
