Table 1.
Optimization of the Domino Cu-Catalyzed Amidation/Cyclization Reaction
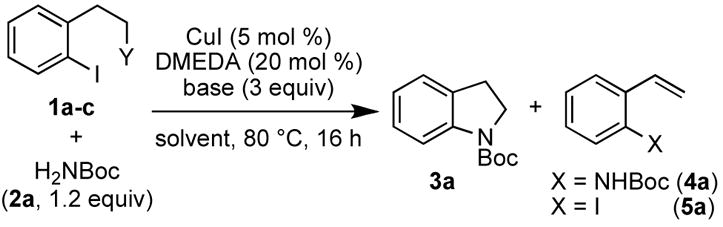 | ||||||
|---|---|---|---|---|---|---|
| entry | Y | base | solvent | yield 3aa | yield 4a | yield 5a |
| 1b | I (1a) | Cs2CO3 | THF | 6% | - | 14% |
| 2 | 1a | Cs2CO3 | THF | 37% | 23% | - |
| 3 | 1a | Cs2CO3 | 1,4-dioxane | 35% | 11% | 6% |
| 4 | 1a | Cs2CO3 | toluene | 3% | - | 11% |
| 5c | 1a | Cs2CO3 | THF | 40% | 31% | - |
| 6 | 1a | K3PO4 | THF | 9% | 23% | 14% |
| 7 | 1a | K2CO3 | THF | - | - | 33% |
| 8 | Cl (1b) | Cs2CO3 | THF | 87% | - | |
| 9 | OMs (1c) | Cs2CO3 | THF | 89%d | - | - |
GC yield with dodecane as an internal standard; 99% conversion of 1, unless indicated otherwise.
Experiment performed at rt; 41% conversion of 1a.
Racemic trans-1,2-N,N′-dimethylcyclohexanediamine was used as a ligand.
Isolated yield.
