Table 2.
Arylation of Anilides with Benzene
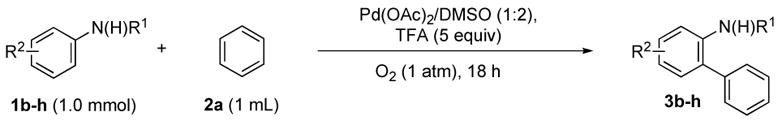 | |||||
|---|---|---|---|---|---|
| entry | anilide | Pd(OAc)2 | temp | biphenyl | yielda |
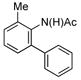
| 1 |
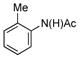 (1b) (1b) |
5 mol% | 80 °C |
|
70% (3b) |
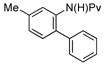
| 2 |
|
5 mol% | 90 °C |
|
91% (3c) |
| 3 |
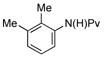 (1d) (1d) |
10 mol% | 90 °C |
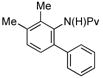
|
84% (3d) |
| 4 |
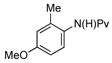 (1e) (1e) |
7.5 mol% | 80 °C |
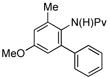
|
87% (3e) |
| 5 |
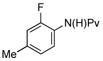 (1f) (1f) |
10 mol% | 90 °C |
|
86% (3f) |
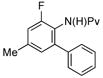
| 6 |
|
10 mol% | 100 °C |
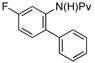
|
68%b (3g) |
| 7 |
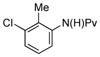 (1h) (1h) |
10 mol% | 55°C |
|
59%a,c (3h) |
Isolated yield, average of two runs.
Incomplete conversion of the starting material.
Reaction run for 96 h.
