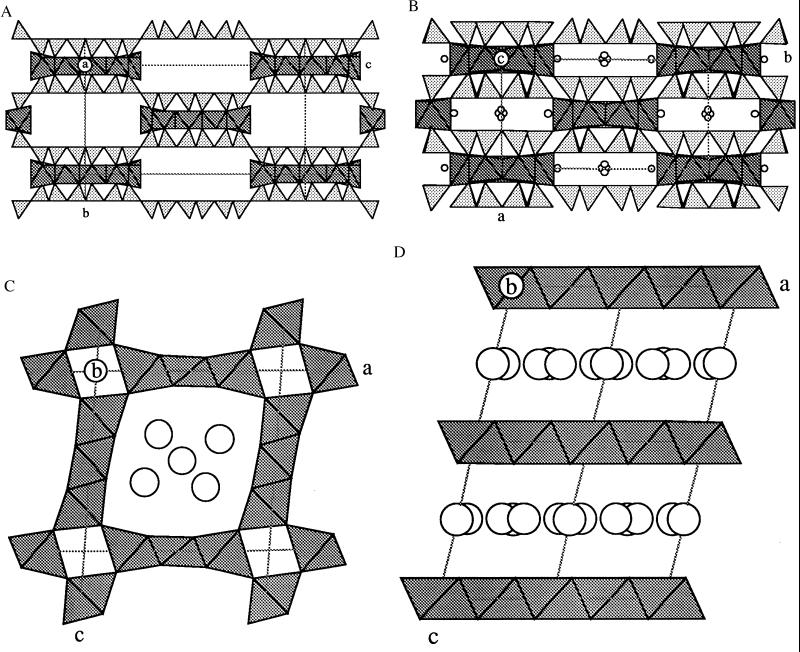
Figure 2.
Representative examples of structures containing Mn (shaded octahedra). Sepiolite and ungarettiite also contain SiO4 tetrahedra (triangles). (A) Sepiolite. Idealized model showing tetrahedral inversion with six “up” and six “down” triangles. The pentuple bands of edge-shared Mg octahedra flare out to accommodate the weaker bonding to water molecules in the rectangular gaps. (B) Ungarettiite, a member of the amphibole mineral family. Refined model showing the inversion of trapezium-shaped bands of silica tetrahedra, and the cross-linking of the triple bands of Mn octahedra. Small circles next to the Mn are Na. Subtle structural complexities are related to the mainly trivalent valency of Mn. (C) Todorokite. Edge-shared triple-octahedral bands are vertex-linked around tetragonal tunnels in this 3 × 3 structure type. There are many stacking variants starting with the 1 × 1 type in rutile. The circles represent positions occupied by Na and water not differentiated in a structural model fitted to x-ray powder diffraction data. (D) Birnessite. An example of Mn octahedral layers linked together by Na and water (undifferentiated circles).