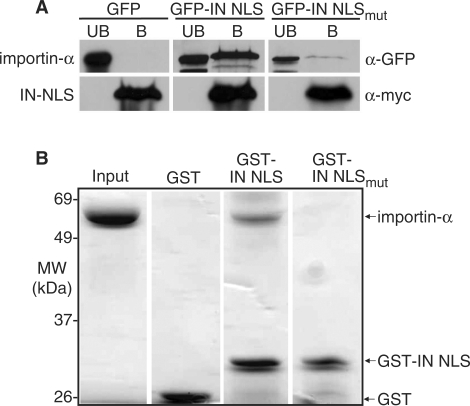
Figure 6.
Ty1 IN interacts with importin-α. (A) IN NLS coimmunoprecipitates with importin-α from whole cell yeast lysate. Yeast cells expressing importin-α-myc were transformed with plasmids expressing GFP (control), GFP2-IN NLS, or GFP2-IN NLSmut. Cell lysates were prepared from induced cultures and epitope-tagged importin-α protein was purified using anti-myc beads as described in the ‘Materials and Methods’ section. GFP proteins present in the bound (B) and unbound (UB) fractions were detected with an anti-GFP antibody. An anti-myc antibody was used to ensure enrichment of importin-α-myc in the bound fraction. (B) Importin-α binds directly to IN NLS in vitro. Purified ΔIBB-importin-α was incubated with GST-IN NLS, or as controls, GST alone or GST-IN NLSmut. GST-fusion proteins and any associated proteins were purified on glutathione sepharose beads as described in the ‘Materials and Methods’ section. Bound fractions were collected and resolved on a 10% SDS-PAGE gel. As a control, 4 μg of purified ΔIBB-importin-α was loaded in the first lane (Input). ΔIBB-importin-α migrates at approximately 70 kDa; GST migrates at approximately 25 kDa; and GST-IN NLS migrates at approximately 35 kDa.