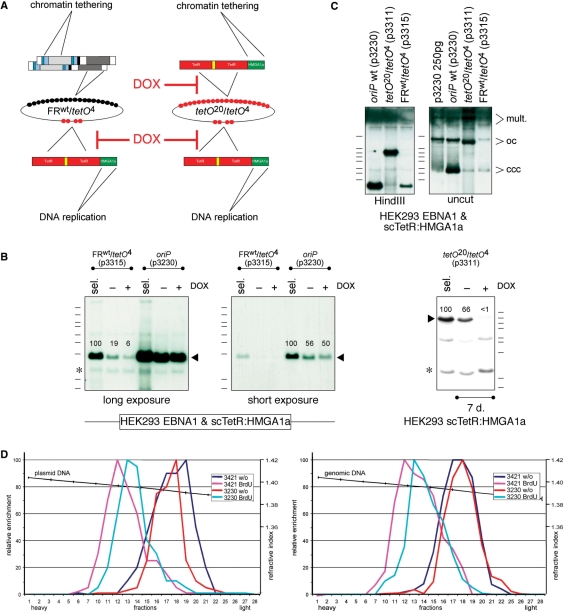
Figure 3.
scTetR:HMGA1a mediates both chromatin tethering and DNA replication. (A) Schematic overview of the hybrid FRwt/tetO4 and synthetic (tetO20/tetO4) replicon plasmids. (B) p3230 (oriP wt), p3315 (FRwt/tetO4) and p3311 (tetO20/tetO4) were stably transfected in HEK293 cells expressing EBNA1 + scTetR:HMGA1a; DNAs were prepared and digested with PmlI and HindIII. In the absence of hygromycin selection, addition of doxycycline (+DOX) caused a modest reduction in the copy number of p3315, but a dramatic loss of p3311; the drug did not affect p3230 copy numbers as indicated by relative signal strengths in percent. Asterisks (*) indicate background signals. (C) p3230 (oriP wt), p3315 (FRwt/tetO4) and p3311 (tetO20/tetO4) were stably introduced (sel.) as described in (B). Low-molecular weight DNAs were prepared, digested with PmlI and HindIII to cleave both plasmid and cellular DNA (left) or PmlI (right) to cleave cellular DNA, only. Southern blot hybridization was performed as in Figure 1C. All plasmids replicated and were maintained extrachromosomally as indicated by signals characteristic of covalently closed circles (ccc) and open circles (oc) DNA molecules together with multimeric forms (mult.). (D) Synthetic, oriP-like plasmids replicate once per cell cycle in Meselson–Stahl density transfer experiments. scTetR:HMGA1a-expressing HEK293 cells were stably transfected with p3230 (oriP wt) or p3421 (tetO20/tetO4) and newly synthesized DNA was labeled with 5-bromodeoxyuridine and separated on CsCl density equilibrium gradients. All DNAs shifted from light, unlabeled DNA (1.70 g/cm3) to heavylight, hemisubstituted DNA (1.73 g/cm3). Plasmid DNA in the gradient fractions (left panel) was quantified by real-time PCR in comparison with chromosomal DNA (right panel). The data indicate that wild-type oriP and synthetic oriP-like plasmids replicated in synchrony with cellular DNA.