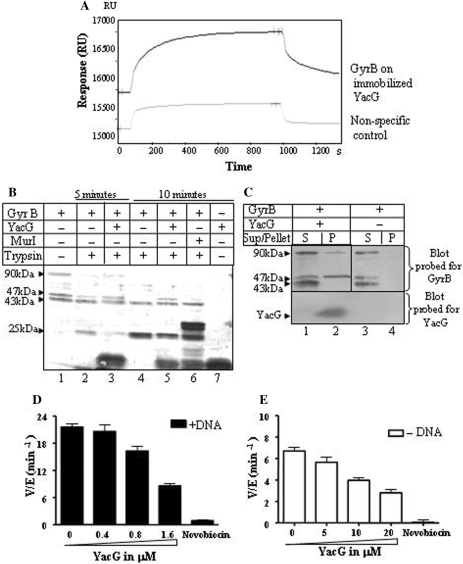
Figure 3.
Interaction between YacG and DNA gyrase subunits and its effect on ATPase activity. (A) SPR refractometry. Black colored line represents response for E. coli GyrB passed over YacG immobilized on CM5 sensor surface, grey colored line represents response for nonspecific control. Interaction was assessed in a buffer as described in Materials and Methods section. Passing 1 μM GyrB over immobilized YacG resulted in increase of 92 RU above nonspecific control. (B): Effect of YacG on trypsin-mediated proteolytic signature of GyrB subunit. 2 μg of GyrB was subjected to partial tryptic digestion: lane 1, undigested GyrB; lanes 2 and 4, GyrB and trypsin; lanes 3 and 5, GyrB, trypsin and YacG; lane 6, GyrB, trypsin and MurI; lane 7, undigested YacG. Duration of digestion: 5 min in lanes 2 and 3, 10 min in lanes 4–6; GyrB preparation (lane 1) used here was a mixture of full length GyrB and two stable fragments (47 kDa CTD and 43 kDa NTD). All these fragments were verified by mass spectrometry. (C) Western blots for the pull-down assay with Ni-NTA sepharose beads. The assays were carried out as described in the Materials and Methods section. Top panel: blot probed with anti-GyrB polyclonal antibodies. Lanes 1 and 2, supernatant and pellet fractions of the mixture of GyrB and YacG pulled down by Ni-NTA sepharose beads; lanes 3 and 4, supernatant and pellet fractions of the GyrB alone pulled down by Ni-NTA sepharose beads; bottom panel, same blot probed for YacG with anti-His tag antibodies. (D and E) Effect of YacG on ATPase activity of DNA gyrase. (D) DNA-stimulated ATPase activity. Reactions were performed with 400 nM each of GyrA and GyrB subunits and 150 μg ml−1 DNA, lane 1, gyrase; lanes 2–4, gyrase with 0.4, 0.8 and 1.6 μM YacG, respectively; lane 5, gyrase and 1 μg/ml novobiocin. (E) Intrinsic ATPase activity. 1.4 μM GyrB subunit was used, DNA and GyrA were omitted; lane 1, GyrB; lanes 2–4, GyrB with 5, 10, 20 μM YacG, respectively; lane 5, GyrB and 1 μg/ml novobiocin. 2 mM ATP present in all the reactions. The average of three independent experiments is depicted graphically.