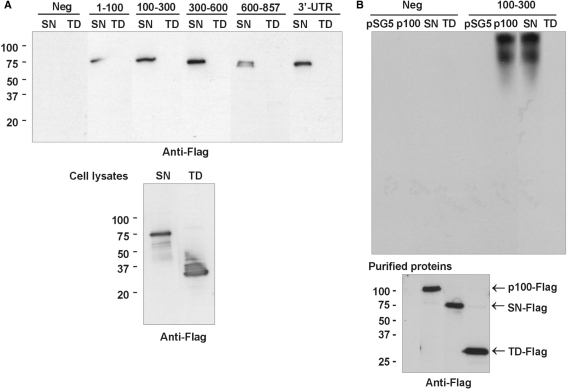
Figure 2.
SN-like domains mediate the p100 interaction with 3′-UTR. (A) Mapping of the binding sites of SN-like domains and TD. The same 3′-UTR transcripts as in Figure 1C were used in the affinity purification reactions with cell lysates prepared from SN-like domains or TD overexpressing COS-1 cells. The results are shown as a western blot of Flag-tagged protein domains bound to transcripts (upper panel). Below is the western blot of Flag-tagged TD and SN-like domains from cell lysates. (B) REMSA. COS-1 cells were transfected with empty pSG5 vector, or with p100-Flag, SN-Flag, or TD-Flag expression constructs. Immunoaffinity-purified Flag-tagged proteins were incubated with biotinylated probes consisting of the segment of luciferase coding region (Neg, four first lanes) or 100–300 probe (four last lanes), as indicated. The unbound probe was digested by RNAase A. The relative levels of purified proteins used in REMSA are shown in anti-Flag western blot (bottom panel).