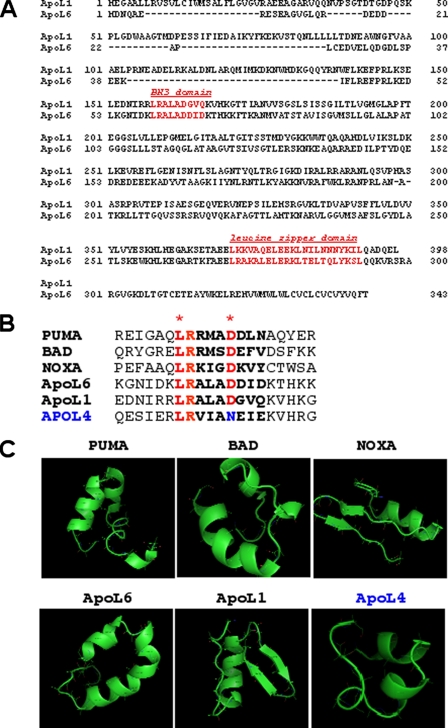
FIGURE 1.
Sequence and domain analysis of human apoL1. A, polypeptide sequence alignment of human apoL1 and apoL6. Over all, apoL1 and apoL6 shows 27% identity and 42% similarity at the aa level. Putative domains in apoL1 include a BH3 domain (aa 158–166) and a leucine zipper domain (aa 365–392). B, sequence alignment of the 20-aa region containing putative BH3 domain in known BH3-only proteins (PUMA, BAD, NOXA, and apoL6) and apoL1 and apoL4. The 9-aa BH3 domain are in boldface and the two amino acids, L and D, which are conserved in all known BH3 domains, are labeled in red with asterisks. ApoL4 does not possess the conserved Asp residue, instead, it is substituted by an Asn (in blue). C, predicted tertiary structure of the 20-aa region containing putative BH3 domain in the indicated proteins. As expected, the 9-aa BH3 domains of known BH3-only proteins, PUMA, BAD, NOXA, and apoL6, show amphipathic α-helical structures. Importantly, the putative BH3 domain of apoL1 also possesses an α-helical domain. In contrast, apoL4 does not possess a complete, canonical α-helical structure in that region.