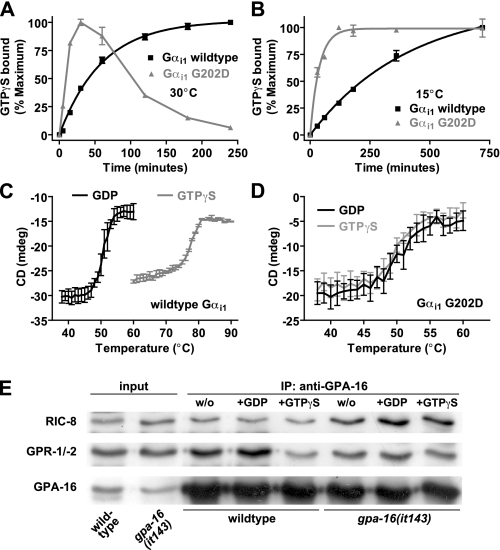
FIGURE 1.
Temperature-dependent nucleotide binding, protein stability, and protein interactions of wild type and G202D Gα subunits. A and B, time course of GTPγS binding by wild type and G202D Gαi1 measured at 30 °C (A) or 15 °C (B). 100 nm Gα was incubated with 1 μm [35S]GTPγS, and bound nucleotide was measured at indicated times. Data were fit to single exponential association curves (95% confidence intervals in brackets) as follows: 30 °C wild type, 0.017 (0.015–0.019) min-1; 30 °C G202D, data could not be fit; 15 °C wild type, 0.0025 (0.0021–0.0028) min-1; 15 °C G202D, 0.027 (0.021–0.032) min-1. C and D, CD in millidegrees (mdeg) of 4.4 μm wild type Gαi1 (C) or G202D Gαi1 (D) was measured at 208 nm in both GDP- and GTPγS-bound conformations. Thermal melting curves were generated by measuring CD values at 1 °C intervals. Data are graphed as mean ± S.E. The mean melting temperatures (S.E. in parenthesis; n = 3) for wild type Gαi1 (C) were GDP 50.2 °C (0.4) and GTPγS 77.2 °C (0.4) and for G202D Gαi1 (D) were GDP 49.5 °C (0.8) and GTPγS 50.1 °C (0.01). E, co-immunoprecipitation conducted at 16 °C using wild type or gpa-16 (it143) embryonic extracts and GPA-16 antibody, either without (w/o) exogenous nucleotides or in the presence of 100 μm GDP or GTPγS. Immunoprecipitated material was analyzed by Western blot using antibodies against RIC-8, GPR-1/-2, or GPA-16. Input corresponds to 1/70 of starting material.