Abstract
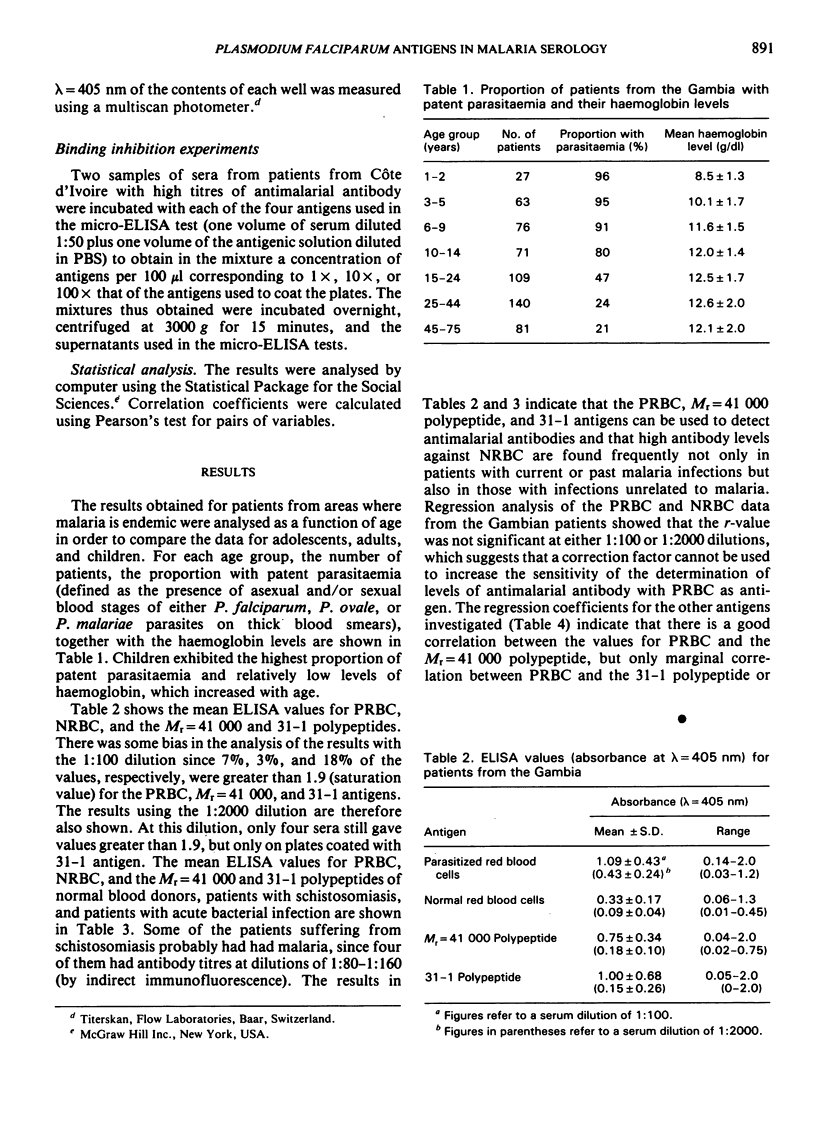
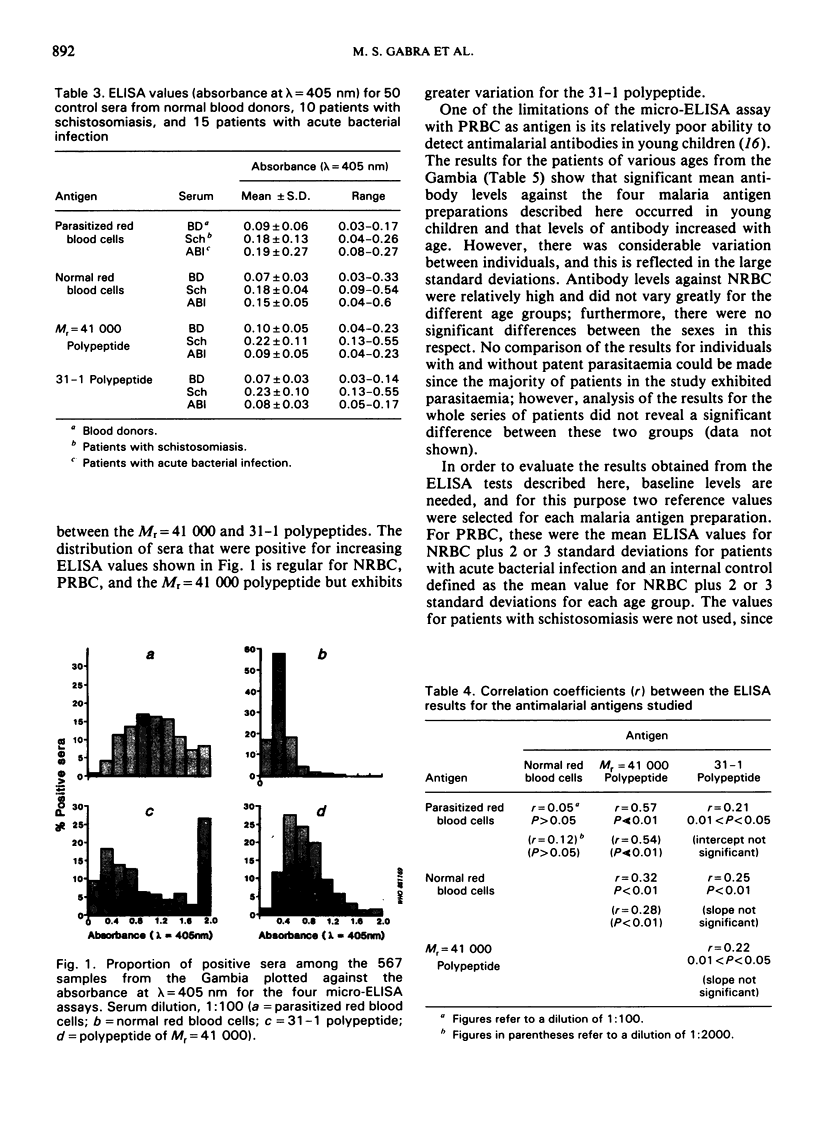
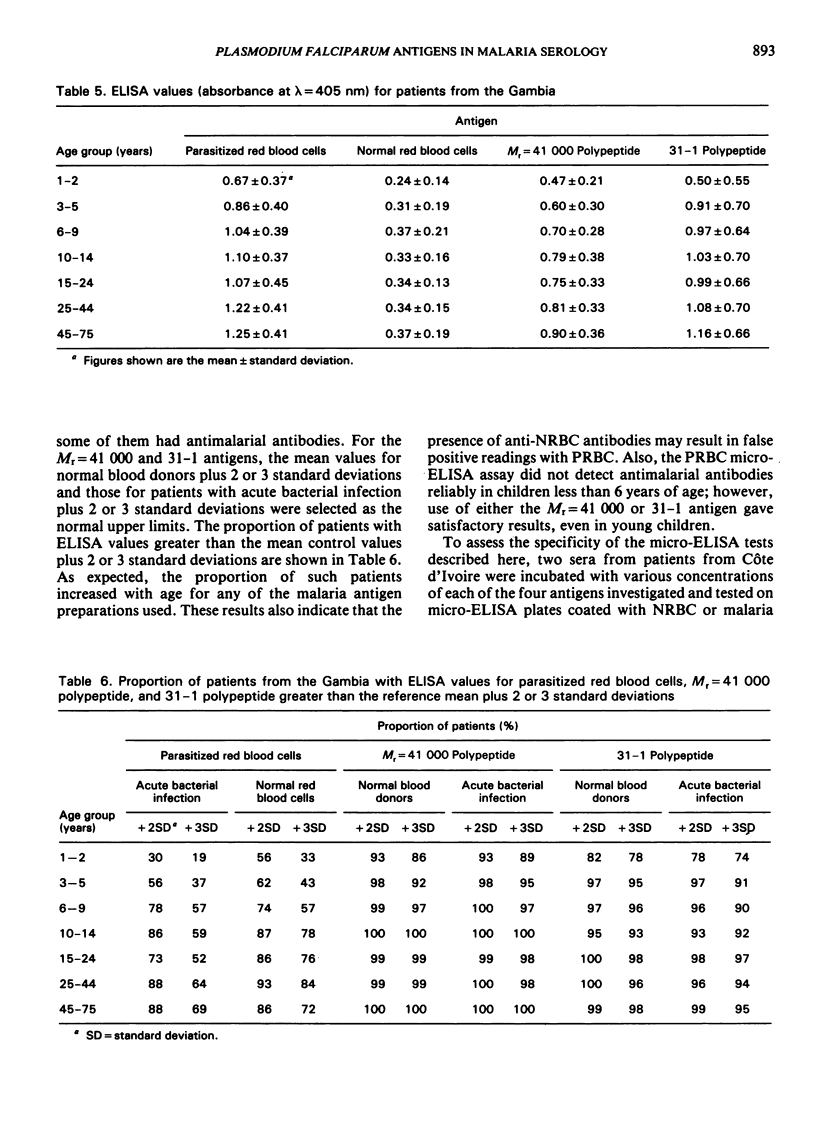
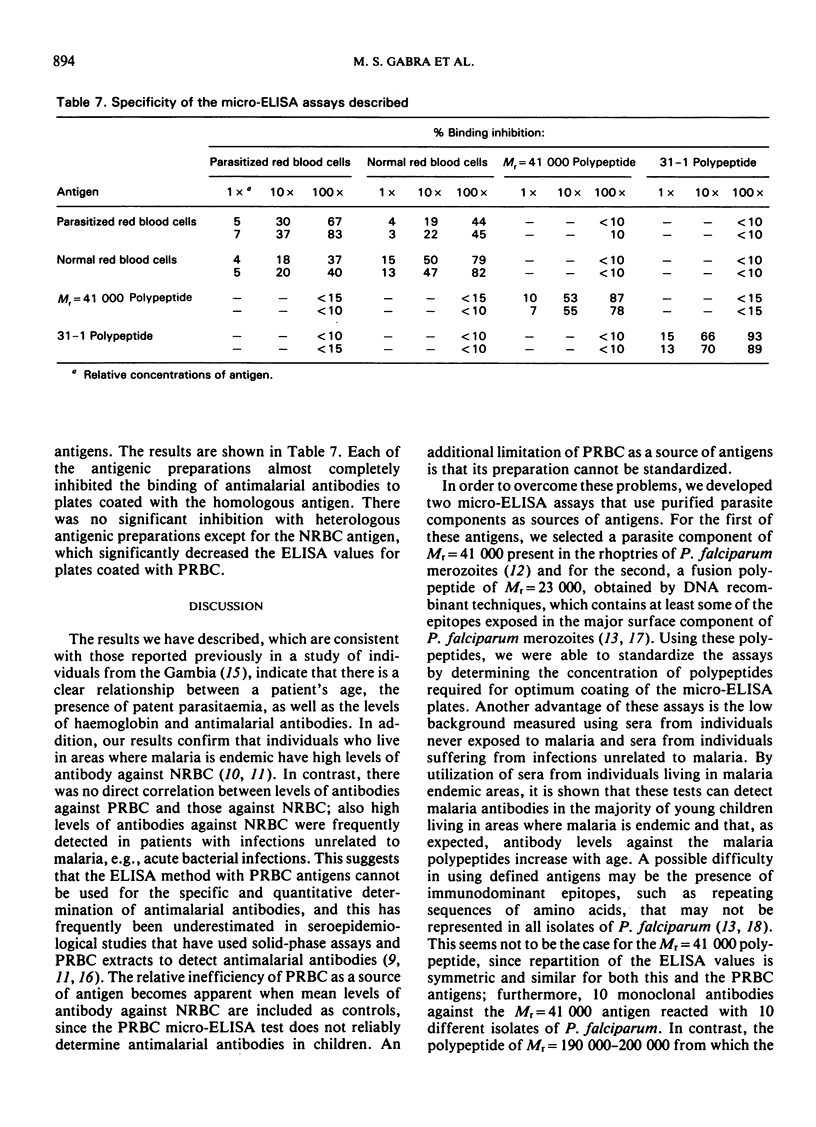
The study evaluates three enzyme-linked immunosorbent assays (ELISA) of malaria antigens suitable for use in large-scale epidemiological studies. Results obtained using sera from 567 persons from the Gambia indicated that the micro-ELISA method using parasitized red blood cell extract did not reliably quantitate antimalarial antibodies, especially in young children. In contrast, two micro-ELISA methods that employed purified, defined antigens (a polypeptide of Mr = 41 000 present in rhoptries, and a 31-1 fusion polypeptide corresponding to a merozoite surface antigen) permitted the precise determination of antimalarial antibodies in both adults and children. Problems and advantages associated with the use of the Mr = 41 000 and 31-1 antigens for the determination of antimalarial antibodies are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Golenser J., Spira D. T., Sulitzeanu D. Plasmodium falciparum: assay of antigens and antibodies by means of a solid phase radioimmunoassay with radioiodinated staphylococcal protein A. Trans R Soc Trop Med Hyg. 1981;75(3):421–425. doi: 10.1016/0035-9203(81)90111-5. [DOI] [PubMed] [Google Scholar]
- Bidwell D. E., Voller A. Malaria diagnosis by enzyme-linked immunosorbent assays. Br Med J (Clin Res Ed) 1981 May 30;282(6278):1747–1748. doi: 10.1136/bmj.282.6278.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. C., Martinez J. M., Collins W. E. Seroepidemiological studies of malaria in pregnant women and newborns from coastal El Salvador. Am J Trop Med Hyg. 1980 Mar;29(2):151–157. doi: 10.4269/ajtmh.1980.29.151. [DOI] [PubMed] [Google Scholar]
- Cheung A., Shaw A. R., Leban J., Perrin L. H. Cloning and expression in Escherichia coli of a surface antigen of Plasmodium falciparum merozoites. EMBO J. 1985 Apr;4(4):1007–1011. doi: 10.1002/j.1460-2075.1985.tb03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Walliker D., Morgan G. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science. 1982 Jul 16;217(4556):254–257. doi: 10.1126/science.6178159. [DOI] [PubMed] [Google Scholar]
- McGregor I. A. Mechanisms of acquired immunity and epidemiological patterns of antibody responses in malaria in man. Bull World Health Organ. 1974;50(3-4):259–266. [PMC free article] [PubMed] [Google Scholar]
- McGregor I. A., Turner M. W., Williams K., Hall P. Soluble antigens in the blood of African patients with severe plasmodium falciparum malaria. Lancet. 1968 Apr 27;1(7548):881–884. doi: 10.1016/s0140-6736(68)90237-7. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Gabra M. S., Stocker J. W., Chizzolini C., Richle R. Immunization with a Plasmodium falciparum merozoite surface antigen induces a partial immunity in monkeys. J Clin Invest. 1985 May;75(5):1718–1721. doi: 10.1172/JCI111881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. B., Strickland G. T., Yang S. L., Whalen G. E. IgM antibodies to red cells and autoimmune anemia in patients with malaria. Am J Trop Med Hyg. 1973 Mar;22(2):146–152. doi: 10.4269/ajtmh.1973.22.146. [DOI] [PubMed] [Google Scholar]
- Spencer H. C., Collins W. E., Skinner J. C. The enzyme-linked immunosorbent assay (ELISA) for malaria. II. Comparison with the malaria indirect fluorescent antibody test (IFA). Am J Trop Med Hyg. 1979 Nov;28(6):933–936. doi: 10.4269/ajtmh.1979.28.933. [DOI] [PubMed] [Google Scholar]
- Sulzer A. J., Wilson M. The fluorescent antibody test for malaria. CRC Crit Rev Clin Lab Sci. 1971;2(4):601–619. doi: 10.3109/10408367109151318. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer W. H. The relevance of research to the global antimalaria programme. Bull World Health Organ. 1977;55(2-3):133–135. [PMC free article] [PubMed] [Google Scholar]


