Abstract
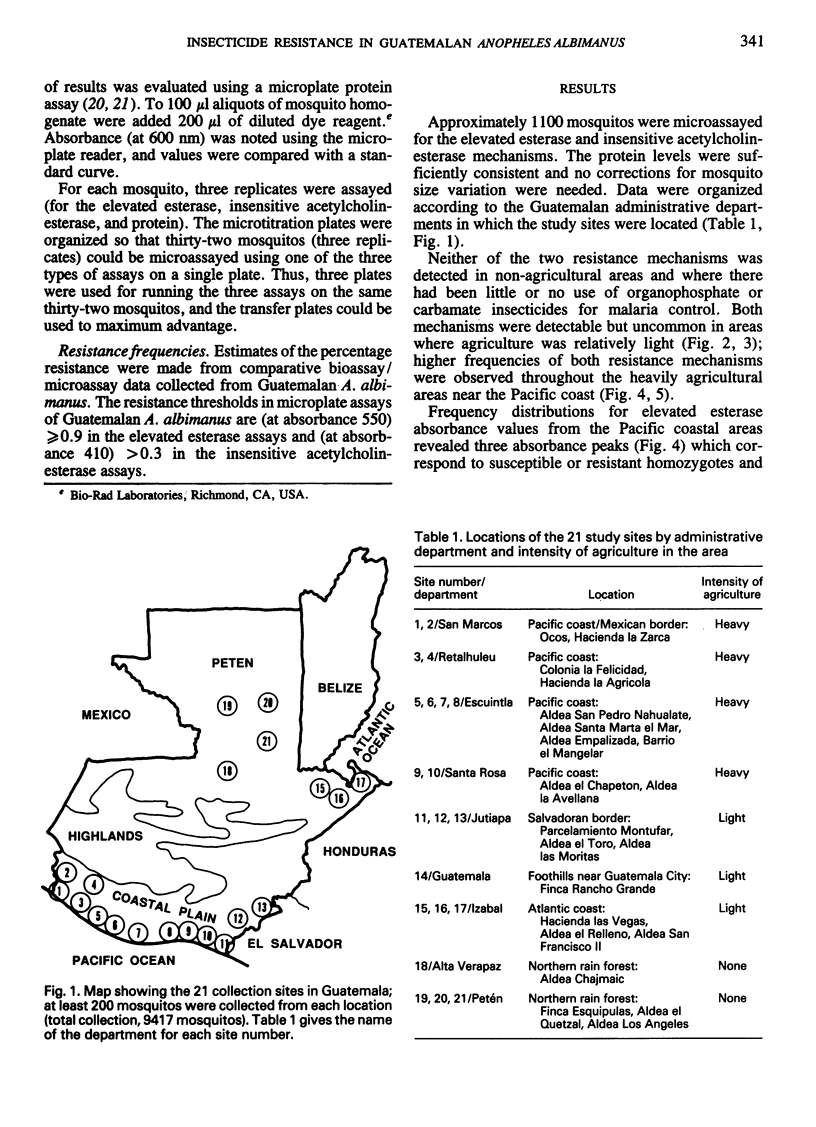
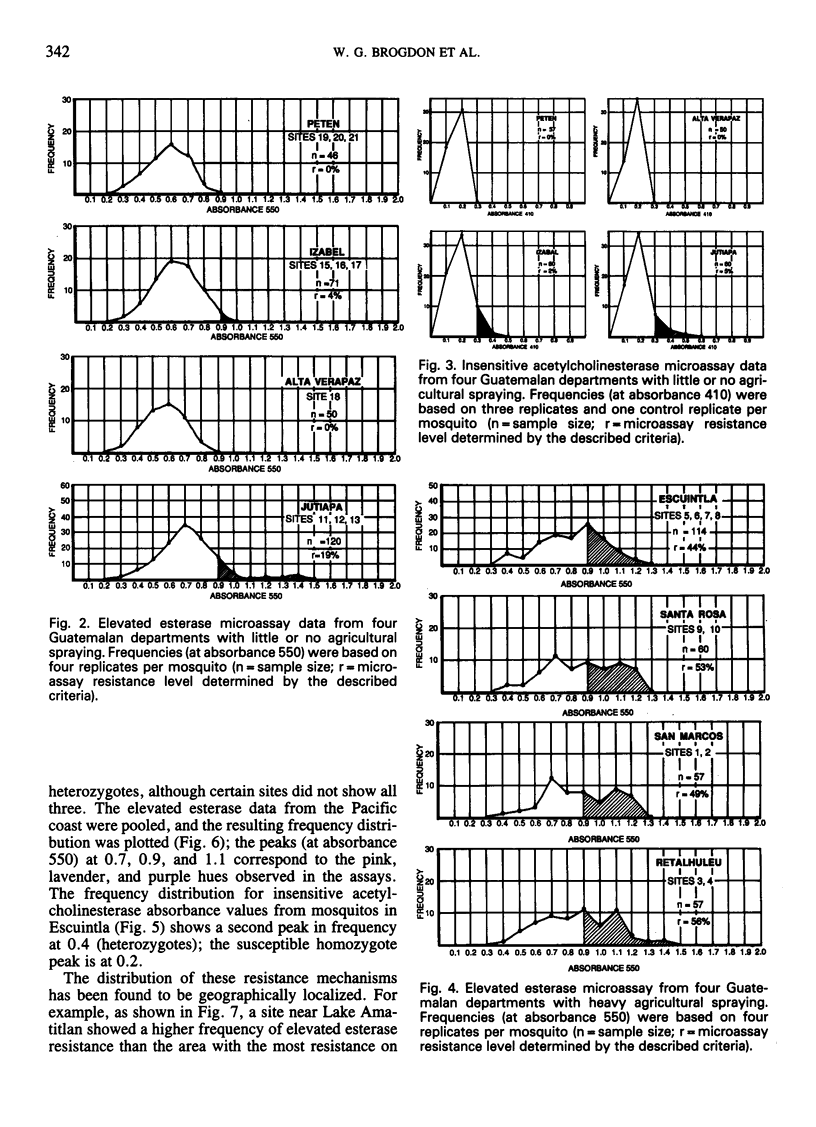
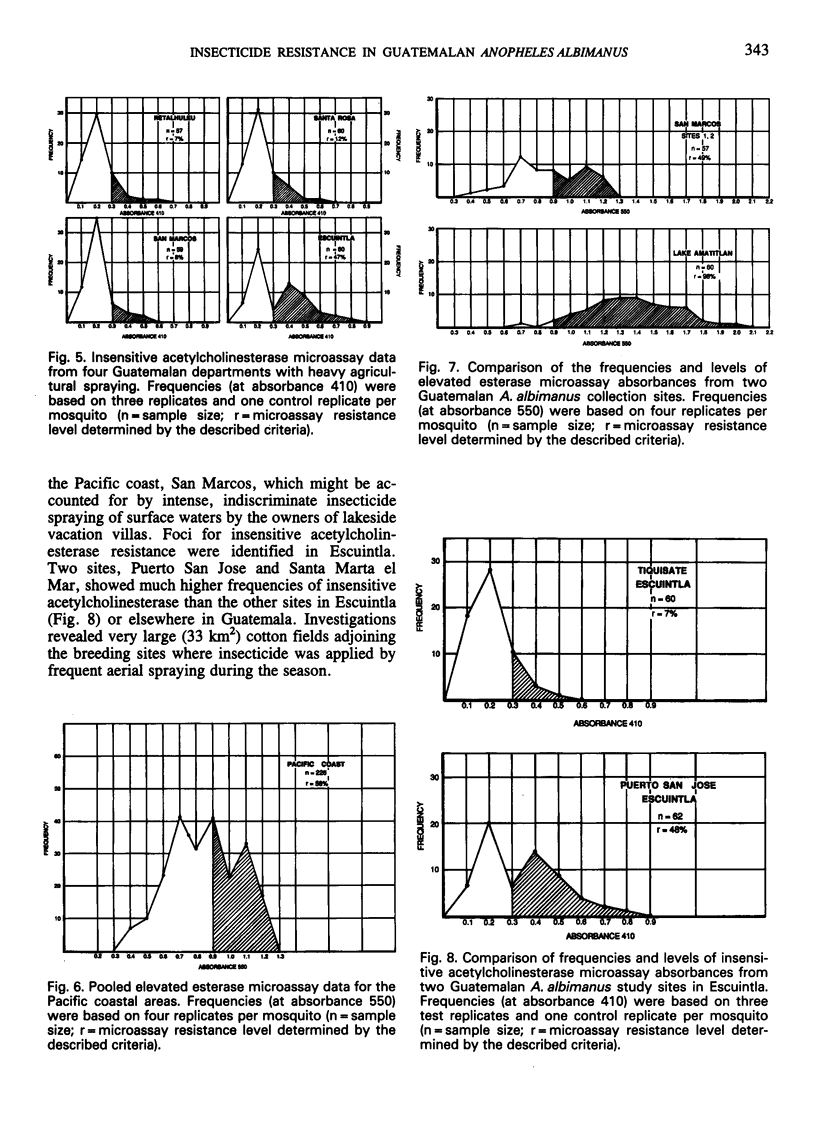
Simple microplate assay methods for determining the frequency of insecticide resistance in single mosquitos were used to study the distribution and localization of organophosphate and carbamate resistance in field populations of Anopheles albimanus Weidemann in Guatemala, where such resistance, caused by heavy use of agricultural pesticides, has long been assumed to be widespread. Areas of complete susceptibility to organophosphates and carbamates were observed, as well as areas where the resistant phenotypes represented up to 98% of the population. Overall, the resistance levels were lower and more localized than expected. Two mechanisms of resistance were identified by the microassay methods. These were the elevated esterase (nonspecific esterase) and insensitive acetylcholinesterase mechanisms which were selected independently, the former (documented for the first time in Central American anophelines) being predominant. These methods represent a promising new technology for the detection and assessment of resistance and will facilitate improved control strategy decisions.
Full text
PDF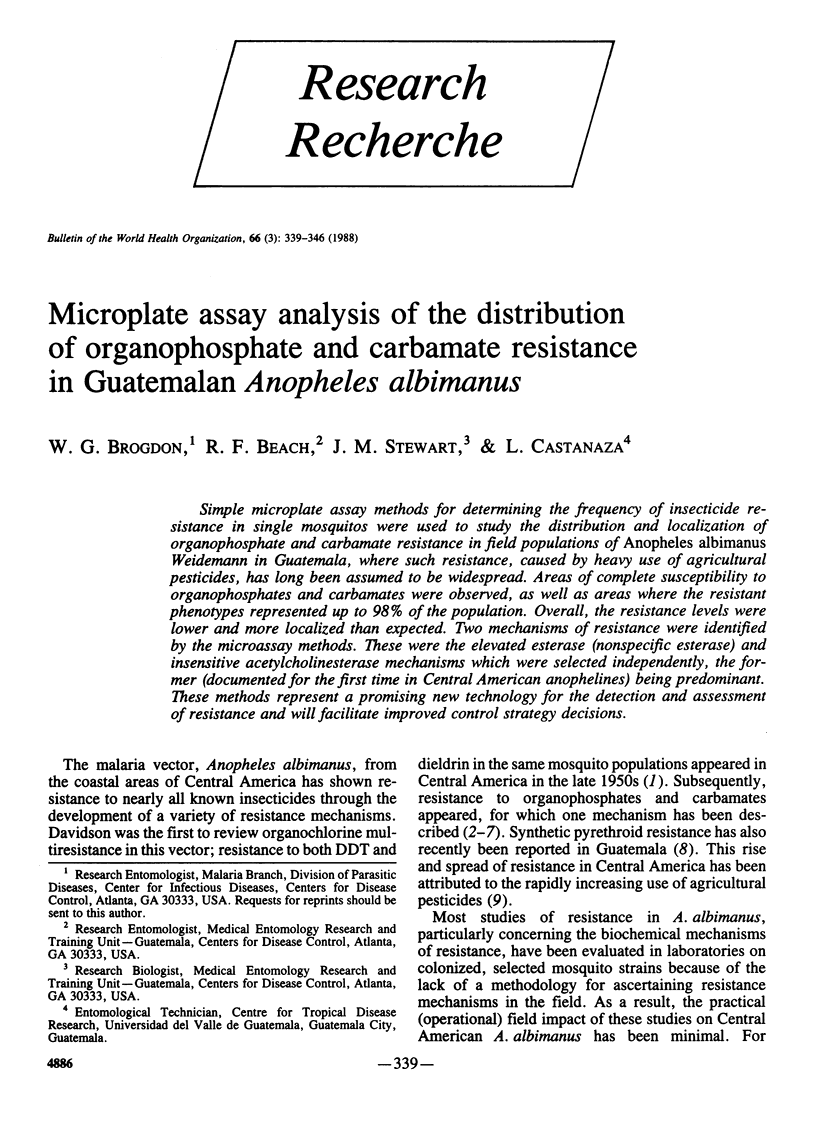




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariaratnam V., Georghiou G. P. Carbamate resistance in Anopheles albimanus. Cross resistance spectrum and stability of resistance. Bull World Health Organ. 1974;51(6):655–659. [PMC free article] [PubMed] [Google Scholar]
- Ayad H., Georghiou P. Resistance to organophosphates and carbamates in Anopheles albimanus Based on reduced sensitivity of acetylcholinesterase. J Econ Entomol. 1975 Jun;68(3):295–297. doi: 10.1093/jee/68.3.295. [DOI] [PubMed] [Google Scholar]
- Breeland S. G., Kliewer J. W., Austin J. R., Miller C. W. Observations on malathion-resistant adults of Anopheles albimanus Wiedemann in coastal El Salvador. Bull World Health Organ. 1970;43(4):627–631. [PMC free article] [PubMed] [Google Scholar]
- Brogdon W. G., Dickinson C. M. A microassay system for measuring esterase activity and protein concentration in small samples and in high-pressure liquid chromatography eluate fractions. Anal Biochem. 1983 Jun;131(2):499–503. doi: 10.1016/0003-2697(83)90204-x. [DOI] [PubMed] [Google Scholar]
- Brown A. W. Insecticide resistance in mosquitoes: a pragmatic review. J Am Mosq Control Assoc. 1986 Jun;2(2):123–140. [PubMed] [Google Scholar]
- Brown T. M., Brogdon W. G. Improved detection of insecticide resistance through conventional and molecular techniques. Annu Rev Entomol. 1987;32:145–162. doi: 10.1146/annurev.en.32.010187.001045. [DOI] [PubMed] [Google Scholar]
- Chapin G., Wasserstrom R. Agricultural production and malaria resurgence in Central America and India. Nature. 1981 Sep 17;293(5829):181–185. doi: 10.1038/293181a0. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Jayawardena K. G., Herath P. R. Pesticide resistance mechanisms produced by field selection pressures on Anopheles nigerrimus and A. culicifacies in Sri Lanka. Bull World Health Organ. 1986;64(5):753–758. [PMC free article] [PubMed] [Google Scholar]
- Mouchès C., Pasteur N., Bergé J. B., Hyrien O., Raymond M., de Saint Vincent B. R., de Silvestri M., Georghiou G. P. Amplification of an esterase gene is responsible for insecticide resistance in a California Culex mosquito. Science. 1986 Aug 15;233(4765):778–780. doi: 10.1126/science.3755546. [DOI] [PubMed] [Google Scholar]
- Sexton J. D., Hobbs J. H., St Jean Y., Jacques J. R. Comparison of an experimental updraft ultraviolet light trap with the CDC miniature light trap and biting collections in sampling for Anopheles albimanus in Haiti. J Am Mosq Control Assoc. 1986 Jun;2(2):168–173. [PubMed] [Google Scholar]


