Abstract
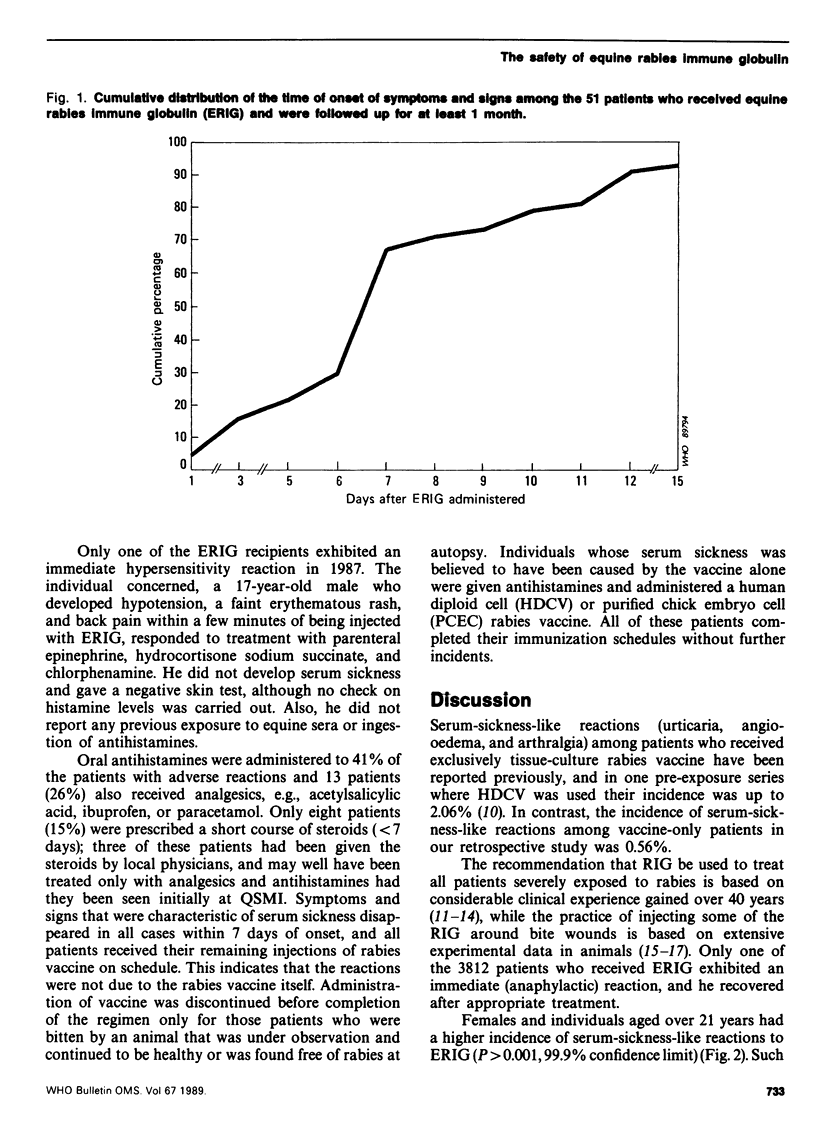
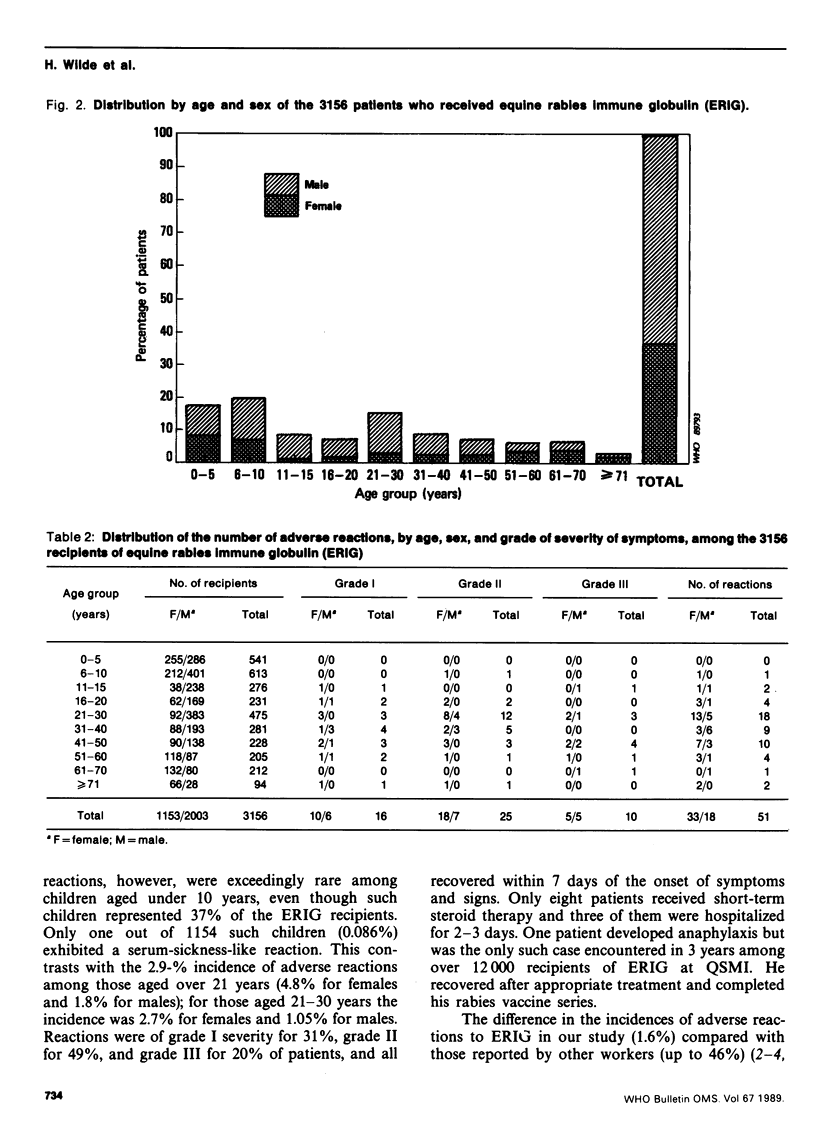
Reported are the results of a retrospective study of 3156 patients who were treated at the Queen Saovabha Memorial Institute, Bangkok, with equine rabies immune globulin (ERIG). Only 51 patients (1.6%) exhibited serum-sickness-like reactions, none of which persisted for more than a week, and only 8 of these patients (15%) were treated with a short course of steroids. One patient, whose skin test was negative, had an immediate anaphylactic reaction to ERIG that responded to parenteral therapy with epinephrine and hydrocortisone sodium succinate. Serum-sickness-like reactions were more frequent among females and over 21-year-olds but were exceedingly rare (0.086%) among children under 10 years of age.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTAZARD M., BAHMANYAR M. Essai pratique du sérum antirabique chez les mordus par loups enragés. Bull World Health Organ. 1955;13(5):747–772. [PMC free article] [PubMed] [Google Scholar]
- HABEL K., KOPROWSKI H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- HOSTY T. S., HUNTER F. R. Incidence of reactions to antirabies horse serum. Public Health Rep. 1953 Aug;68(8):789–791. [PMC free article] [PubMed] [Google Scholar]
- Helmick C. G., Johnstone C., Sumner J., Winkler W. G., Fager S. A clinical study of Merieux human rabies immune globulin. J Biol Stand. 1982 Oct;10(4):357–367. doi: 10.1016/s0092-1157(82)80013-9. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C. Immune sera and antiglycoprotein monoclonal antibodies inhibit in vitro cell-to-cell spread of pathogenic rabies viruses. J Virol. 1987 Oct;61(10):3314–3318. doi: 10.1128/jvi.61.10.3314-3318.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanuphak P., Khawplod P., Sirivichayakul S., Siriprasomsub W., Ubol S., Thaweepathomwat M. Humoral and cell-mediated immune responses to various economical regimens of purified Vero cell rabies vaccine. Asian Pac J Allergy Immunol. 1987 Jun;5(1):33–37. [PubMed] [Google Scholar]
- VEERARAGHAVAN N., SUBRAHMANYAN T. P. Value of antirabies vaccine with and without serum against severe challenges. Bull World Health Organ. 1960;22:381–391. [PMC free article] [PubMed] [Google Scholar]
- Wilde H., Chomchey P., Prakongsri S., Punyaratabandhu P. Safety of equine rabies immune globulin. Lancet. 1987 Nov 28;2(8570):1275–1275. doi: 10.1016/s0140-6736(87)91885-x. [DOI] [PubMed] [Google Scholar]
- Wilde H., Chomchey P., Prakongsri S., Puyaratabandhu P., Chutivongse S. Adverse effects of equine rabies immune gobulin. Vaccine. 1989 Feb;7(1):10–11. doi: 10.1016/0264-410x(89)90003-0. [DOI] [PubMed] [Google Scholar]


