Abstract
The frequency of antrum-type mucosa and gastrin expression in gastric biopsies from the incisura angularis was assessed in 60 consecutive patients having gastrointestinal symptoms. Following the recommendations from the updated Sydney System for the classification and grading of gastritis, two biopsies were taken from the antrum, one from the incisura and two from the corpus. Sections were stained with H&E, Giemsa and for gastrin. Gastrin-positive cells were semi-quantified as: 0 (none), ≤ 9, 10- 49 cells and ≥50 gastrin-labelled cells/40× field. Antrum-type mucosa at the incisura (called antralization) occurred in 30% of the biopsies without inflammation, but in 69% of those with H. pylori-induced gastritis, and in 64% of those with autoimmune gastritis. At the incisura, gastrin-labelled cells (≥10) were found in 62% (18/29) of biopsies showing antralization, but in only 20% (3/15) of those having transitional-type mucosa (p<0.05) and in none of the 16 biopsies having fundic-type mucosa. The similarity in gastrin expression between the mucosa of the gastric antrum and the antral-type mucosa at the incisura substantiates the notion that antralization is a metaplastic transformation. The significantly higher frequency of antral-type mucosa at the incisura in patients with gastritis than in those without gastritis strongly suggests that chronic inflammation per se triggers antralization of the incisura, irrespective of the presence or absence of H. pylori infection.
Keywords: Antralization, incisura, gastrin, chronic gastritis
Introduction
The stomach is anatomically subdivided into four segments: the cardia, the fundus, the corpus or body and the antrum. The frontal profile of the stomach is delineated by the lesser and the greater curvatures [1]. The lesser curvature extends from the cardia to the pyloric sphincter and displays an abrupt change in spatial direction from vertical to horizontal. The vertical (proximal) section limits the medial aspect of the fundus-corpus and the horizontal (distal), the medial aspect of the antrum pylori. The angle in the lesser curvature moulded by the vertical and the horizontal sections is called incisura angularis [1].
The fundic (fundus-corpus) mucosa is built of parietal cells/chief cells while the mucosa of the antrum is built of mucin-producing cells and specialized endocrine cells. The mucosa of the incisura angularis is comprised of an admixture of corpus and antral mucosa [2], hence intermediate mucosa.
About 50% of the entire endocrine cell population in the antrum are gastrin-producing (G cells). G cells are most common in the neck region just below the pits [2]. Gastrin antibodies detect cytoplasmic granules located between the nucleus and the basement membrane. Gastrin is produced in response to stimuli such as stomach distension, vagal stimulation (mediated by bombesin), presence of luminal amino acids and hypercalcemia. The release of gastrin is inhibited by increased intragastric acidity. Similar endocrine cells are not present in the fundic mucosa [2–4]. Antral gastrin stimulates parietal cells to produce hydrochloric acid (HCl). This is done indirectly by binding CCK2/gastrin receptors on endocrine-like cells (ELC) through the release of histamine. Acting in a paracrine fashion, parietal cells are stimulated to secrete H+ ions. Gastrin also causes chief cells to secrete pepsinogen, which is converted into pepsin under the influence of a high HCL production and of pancreatic enzymes from acinar cells [2–4].
Endoscopical gastric biopsies are usually taken in symptomatic patients to assess the histological state of the gastric mucosa. This method permits to diagnose gastritis (grade of inflammation), Helicobacter pylori infection and gastritis sequels, such as mucosal atrophy, pseudopyloric metaplasia and intestinal metaplasia [5–18].
Four biopsies are usually taken, two from the antrum and two from the corpus. The updated Sydney System for the classification and grading of gastritis [19] recommends to include, in addition to the four aforementioned sites, a fifth biopsy from the incisura angularis.
More recently, Xia et al [20–23] studied mucosal changes occurring at the incisura angularis. They classified the mucosa of the incisura angularis into i) antral-type, with mucous-secreting pyloric glands (called antralization of the mucosa of the incisura); ii) transitional-type with mixed antrum-body-glands (called junctional mucosa) and corpus/body-type with acid-secreting or oxyntic glands.
Gastrin is a marker of the pyloric mucosa [2]. No studies have been carried out to explore the presence of this hormone in the various mucosal subtypes occurring at the incisura angularis [20–23]. In this work, we investigated the occurrence of gastrin-producing cells in the antrum, the incisura and the corpus, with particular attention to the presence of gastrin-expressing cells in the various mucosal subtypes at the incisura angularis.
Material and Methods
Specimen Collection
Gastric biopsies were obtained in 60 patients having gastrointestinal symptoms. Patients receiving H2 receptor blockers, on PPI therapy or on nonsteroid anti-inflammatory drug (NSAID) at entry were excluded. The Regional Ethical Committee approved the study.
Gastric biopsies were taken from 5 different sites, following the recommendations from the updated Sydney System for the classification and grading of gastritis [19]: two from the antrum, one from the incisura angularis and two from the corpus.
Special Stains
Biopsies were fixed in 4% buffered formalin and processed for paraffin embedment. Sections were routinely stained with hematoxylin-eosin (H&E) and Giemsa. Immunohistochemical stain with gastrin (DAKO, Glistrup, Denmark) antibody (dilution at 1:4000) was performed using the standard protocol with DAB as a detector and appropriate positive control.
A high-power field (40×) containing a priori most gastrin-labelled cells was chosen to assess the number of gastrin-positive mucosa cells. The frequency of gastrin-positive cells/40× field was semi-quantified as follows: no labelled cells: 0; ≤ 9 labelled cells; 10- 49 labelled cells and ≥50 labelled cells.
After grading the immunoreactions, the histological make-up of the incisura angularis was classified in H&E sections, blind to the results of the immunohistochemical reactions into the 3 subtypes proposed by Xia et al [20, 21, 23].
Statistical Analysis
The non-parametric test of Wilcoxon was performed using StatView Version 4.5 software (Abacus Concepts, Berkley, CA, USA). Statistical significance was defined as p<0.05.
Results
Among the 60 patients, 33 were females and 27 were males. The mean age was 58.6 years, ranging from 18 to 89 years.
Histological examination revealed that 30 patients had normal mucosa, 16 had H. pylori-induced gastritis and 14 had autoimmune gastritis (Table 1). Autoimmune gastritis is an inflammatory condition of the stomach that is associated with autoantibodies to parietal cells and intrinsic factor and can lead in a small percentage of patients to destruction of the oxyntic mucosa, pernicious anemia, and the development of carcinoid tumors that are typically indolent. Autoimmune gastritis is also referred to as Type A gastritis and is recognized as a corpus-restricted atrophic gastritis in the updated Sydney classification system [19].
Table 1.
Antralization of the mucosa at the incisura angularis
| Mucosa at the incisura angularis | ||||
|---|---|---|---|---|
| Antral-type | Transitional-type | Corpus-type | Total | |
| Normal | 9 (30%) | 11 (36.7%) | 10 (33.3%) | 30 (100%) |
| H. Pylori Gastritis | 11 (68.8%) | 2 (12.5%) | 3 (18.7%) | 16 (100%) |
| Autoimmune gastritis | 9 (64.3%) | 2 (14.3%) | 3 (21.4%) | 14 (100%) |
| Total | 29 (48.3%) | 15 (25.0%) | 16 (26.7%) | 60 (100%) |
The mean age in the 30 patients having histologically normal gastric mucosa was 56.6 years (range: 18–80 years), 60.8 years (range 27–89) in the 16 patients with H. pylori gastritis, and 64.1 years (range 26–89 years) in the 14 patients with autoimmune gastritis. The difference was not significant (p< 0.6).
The mean age of patients having antral-type mucosa at the incisura was 63.2 years (range 27–89 years), 52.2 years (range 26–76 years) in patients having transitional-type mucosa and 60.0 years (range 35–89 years) for those having fundic-type mucosa. Although the mean age for patients having antral mucosa at the incisura was higher than those having transitional-type mucosa, the difference was not significant (p<0.6).
The histological finding of mucosa at the incisura is summarized in Table 1. Among the 60 biopsies from the incisura, 48% had antrum-type mucosa only, 25% had intermediate-type mucosa and the remaining 27% had fundic-type mucosa only.
Antralization of the incisura occurred in 30% of the 29 patients having normal gastric mucosa, in 69% of the 16 patients with H. pylori-induced gastritis, and in 64% of the 14 patients with autoimmune gastritis (Table 1). The difference between antralization of the incisura angularis in patients without inflammation and those with inflammation (H. pylori-induced gastritis and autoimmune gastritis) was significant (p<0.05).
The frequency of gastrin-labelled cells in the mucosa of the antrum and body is summarized in Table 2. Among the 60 antral biopsies, 45% had ≥50 gastrin-labelled cells (Figure 1), 50% had 10 to 49 gastrin-labelled cells, and the remaining 5% had ≤9 gastrin-labelled cells. In contrast, only 6 or 10% of the 60 biopsies from the fundic mucosa contained ≤9 gastrin-labelled cells. The 6 biopsies were all from patients with autoimmune gastritis. The remaining 54 biopsies from the fundic mucosa showed no gastrin-labelled cells.
Table 2.
Gastrin expression in the mucosa of the antrum and body
| Histological phenotype | No labelled cells | ≤ 9 labelled cells | 10–49 labelled cells | ≥50 labelled cells | Total |
|---|---|---|---|---|---|
| Antrum | 0 | 3 (5.0%) | 30 (50%) | 27 (45%) | 60 (100%) |
| Body | 54 (96.0%) | 6 (10.0%%) | 0 | 0 | 60 (100%) |
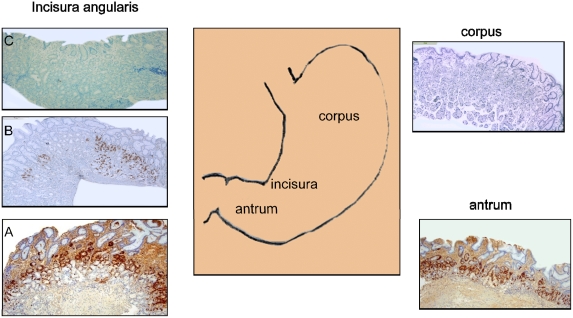
Figure 1.
Diagram of the stomach anatomy (middle panel) with positive and negative expression of gastrin in the antrum and corpus, respectively (right panel). Representative images showing strong expression of gastrin in the antrum-type mucosa (A), discontinuous expression of gastrin in the transitional-type mucosa (B) and absent expression of gastrin in the fundic-type mucosa at the incisura (C). (gastrin immunostains, all at 4X magnifications).
5% (n=3) of the 60 biopsies from the antrum and 10% (n=6) from the fundic mucosa had ≤9 gastrin-labelled cells, whereas 38% (n=11) of the 29 biopsies from the incisura with antrum-type mucosa had ≤9 gastrin-labelled cells. The difference was significant (p<0.05).
The frequency of gastrin-labelled cells in the mucosa of the incisura is summarized in Table3. Among the 29 biopsies from the incisura with antrum-type mucosa, 34% (n=10) had ≥50 gastrin-labelled cells. In contrast, only 7% (n=1) of the 15 biopsies from the incisura with transitional-type mucosa had ≥50 gastrin-labelled cells and in none (=0) of the 16 biopsies with fundic-type mucosa at the incisura (p<0.05) (Figure 1). Similarly, 62% (n=18) of the 29 biopsies from the incisura having antrum-type mucosa had ≥10 gastrin-labelled cells, while only 20% (n=3) of the 15 biopsies from the incisura having transitional-type mucosa had ≥10 gastrin-labelled cells (p<0.05). None of the 16 biopsies from the incisura having fundic-type mucosa had gastrin-labelled cells.
Table 3.
Gastrin expression in the mucosa of the incisura angularis
| Mucosa at the incisura angularis | ||||
|---|---|---|---|---|
| Gastrin | Antral-type | Transitional-type | Fundic-type | Total |
| No labelled cells | 2 | 1 | 16 | 19 (31.7%) |
| ≤ 9 labelled cells | 9 | 11 | 0 | 20 (33.3%) |
| 10- 49 labelled cells | 8 | 2 | 0 | 10 (16.7%) |
| ≥50 labelled cells | 10 | 1 | 0 | 11 (18.3%) |
| Total | 29 (48.3%) | 15 (25.0%) | 16 (26.7%) | 60 (100%) |
Discussion
Several authors have investigated the relationship between the mucosa of the incisura angularis and H. pylori infection. Dursun et al [25] reported that the highest sensitivity in detecting H. pylori infection occurred in biopsies taken the antrum and from the incisura. On the other hand, Ola et al [26] found no benefit in taking additional biopsies from the incisura angularis in assessing H. pylori infection. Sheng-Lian et al [15] reported that the fundic mucosa at the incisura from patients with bile reflux had more severe active inflammation, chronic inflammation, intestinal metaplasia, atrophy or H. pylori infection than in control patients without reflux. Stolte et al [24] found that 58% of 328 gastric biopsies from the incisura contained antral mucosa, 24% intermediate mucosa and 18% corpus mucosa. According to these authors, biopsies showing antral mucosa were exclusively associated with more severe H. pylori gastritis in both the incisura and the antrum, than in those having corpus mucosa only. Xia et al [20, 21, 23] investigated antralization of the incisura angularis and of the body mucosa (known as pseudopyloric metaplasia of the body). These authors [20, 21] claimed that under the influence of H. pylori, the hyperplastic mucous neck cells moved both upwards and particularly downwards in the oxyntic tubules, replacing the specialized parietal and chief cells in the body and the incisura, creating a mucous cell lineage (ulcer-associated cell lineage, UACL).
Similar other studies [21–23], we found here that the histological make-up of the mucosa of the incisura varied. 48% had antral-type mucosa exclusively, 25% had intermediate mucosa and the remaining 27% had corpus-type mucosa exclusively.
Antralization at the incisura concurred with a significantly higher expression of gastrin-producing cells, than intermediate and fundic-type mucosas. The similarity in gastrin-expression between the gastric mucosa of the antrum and the antral-type mucosa at the incisura strongly suggests that antralization of the incisura is conveyed by metaplastic transformation.
Metaplasia is usually defined as the reversible replacement of one differentiated cell type by another mature differentiated cell type. Whether the metaplastic antralization of the mucosa of the incisura is reversible remains to be elucidated.
The frequency of antralization of the incisura was similarly high in patients with H. pylori gastritis and with autoimmune gastritis. These results strongly suggest that chronic inflammation per se plays a causative role in the antralization of the gastric mucosa at the incisura angularis, irrespective of the presence or absence of H. pylori infection.
Acknowledgments
This study was supported by a grant from the Karolinska Institute, Stockholm, Sweden.
References
- 1.Borley NR, Standing Susan. Gray's Anatomy. 39th edition. Philadelphia: Elsevier, Churchill and Livingstone; 2005. Stomach and abdominal esophagus; pp. 1143–1165. Chapter 71. [Google Scholar]
- 2.Owen DA. Stomach. In: Stenrberg Stephen S., editor. Histology for Pathologist. 2nd edition. Philadelphia: Lippincott Raven; 1997. pp. 481–493. Chapter 20. [Google Scholar]
- 3.Kottler RE, Van Niekerk JP, Fouche RF. Lesser curve shortening in gastric ulceration. S Afr Med J. 1982;62:30–32. [PubMed] [Google Scholar]
- 4.Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, Fox JG, Wang TC. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–1983. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342–345. doi: 10.1016/s0016-5107(94)70067-2. [DOI] [PubMed] [Google Scholar]
- 6.Zullo A, Rinaldi V, Hassan C, Lauria V, Attili AF. Gastric pathology in cholecystectomy patients: role of Helicobacter pylori and bile reflux. J Clin Gastroenterol. 1998;27:335–338. doi: 10.1097/00004836-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Satoh K. Does eradication of Helicobacter pylori reverse atrophic gastritis or intestinal metaplasia? Data from Japan. Gastroenterol Clin North Am. 2000;29:829–835. doi: 10.1016/s0889-8553(05)70150-3. [DOI] [PubMed] [Google Scholar]
- 8.el-Zimaity HM. Accurate diagnosis of Helicobacter pylori with biopsy. Gastroenterol Clin North Am. 2000;29:863–839. doi: 10.1016/s0889-8553(05)70153-9. [DOI] [PubMed] [Google Scholar]
- 9.Elitsur Y, Lawrence Z, Triest WE. Distribution of Helicobacter pylori organisms in the stomachs of children with H. pylori infection. Hum Pathol. 2002;33:1133–1135. doi: 10.1053/hupa.2002.129201. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama Y, Horiuchi A, Kumagai T, Kubota S, Kobayashi M, Sano K, Ota H. Discrimination of normal gastric mucosa from Helicobacter pylori gastritis using standard endoscopes and a single observation site: studies in children and young adults. Helicobacter. 2004;9:95–99. doi: 10.1111/j.1083-4389.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 11.Gulmann C, Rathore O, Grace A, Hegarty H, O'Grady A, Leader M, Patchett S, Kay E. ‘Cardiac-type’ (mucinous) mucosa and carditis are both associated with Helicobacter pylori-related gastritis. Eur J Gastroenterol Hepatol. 2004;16:69–74. doi: 10.1097/00042737-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson NK, Färkkilä MA, Voutilainen ME, Arkkila PE. The clinical value of taking routine biopsies from the incisura angularis during gastroscopy. Endoscopy. 2005;37:532–536. doi: 10.1055/s-2005-861311. [DOI] [PubMed] [Google Scholar]
- 13.Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50:1517–1522. doi: 10.1007/s10620-005-2871-7. [DOI] [PubMed] [Google Scholar]
- 14.Farinati F, Cardin R, Russo VM, Busatto G, Franco M, Falda A, Mescoli C, Rugge M. Differential effects of Helicobacter pylori eradication on oxidative DNA damage at the gastroesophageal junction and at the gastric antrum. Cancer Epidemiol Biomarkers Prev. 2004;13:1722–1728. [PubMed] [Google Scholar]
- 15.Sheng-Liang C, Kiang-Zhong M, Zhi-Jun C, Xiao-Yu C, Shu-Dong X. Effects of bile reflux on gastric mucosal lesions in patients with dyspepsia or chronic gastritis. World J Gastroenterol. 2005;11:2834–2837. doi: 10.3748/wjg.v11.i18.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio CA. My approach to reporting a gastric biopsy. J Clin Pathol. 2007;60:160–166. doi: 10.1136/jcp.2006.039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio CA, Jónasson J, Nesi G, Mandai K, Pisano R, King A, Owen D. Extensive intestinal metaplasia in gastric carcinoma and in other lesions requiring surgery: a study of 3,421 gastrectomy specimens from dwellers of the Atlantic and Pacific basins. J Clin Pathol. 2005;58:1271–1277. doi: 10.1136/jcp.2005.029587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques T, David L, Reis C, Nogueira A. Topographic expression of MUC5AC and MUC6 in the gastric mucosa infected by Helicobacter pylori and in associated diseases. Pathol Res Pract. 2005;201:665–672. doi: 10.1016/j.prp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Dixon MF, Genta RM, Yardley JH, Correa P. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last? The International Workshop on the Histopathology of Gastritis. Helicobacter. 1997;2(Suppl 1):S17–24. doi: 10.1111/j.1523-5378.1997.06b09.x. [DOI] [PubMed] [Google Scholar]
- 20.Xia HH, Kalantar JS, Talley NJ, Wyatt JM, Adams S, Chueng K, Mitchell HM. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia? Am J Gastroenterol. 2000;95:114–121. doi: 10.1111/j.1572-0241.2000.01609.x. [DOI] [PubMed] [Google Scholar]
- 21.Xia HH, Zhang GS, Talley NJ, Wong BC, Yang Y, Henwood C, Wyatt JM, Adams S, Cheung K, Xia B, Zhu YQ, Lam SK. Topographic association of gastric epithelial expression of Ki-67, Bax, and Bcl-2 with antralization in the gastric incisura, body, and fundus. Am J Gastroenterol. 2002;97:3023–3031. doi: 10.1111/j.1572-0241.2002.07120.x. [DOI] [PubMed] [Google Scholar]
- 22.Xia HH, Lam SK, Wong WM, Hu WH, Lai KC, Wong SH, Leung SY, Yuen ST, Wright NA, Wong BC. Antralization at the edge of proximal gastric ulcers: does Helicobacter pylori infection play a role? World J Gastroenterol. 2003;9:1265–1269. doi: 10.3748/wjg.v9.i6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia HH, Wong BC, Zhang GS, Yang Y, Wyatt JM, Adams S, Cheung K, Lam SK, Talley NJ. Antralization of gastric incisura is topographically associated with increased gastric epithelial apoptosis and proliferation, but not with CagA seropositivity. J Gastroenterol Hepatol. 2004;19:1257–1263. doi: 10.1111/j.1440-1746.2004.03489.x. [DOI] [PubMed] [Google Scholar]
- 24.Stolte M, Müller H, Talley NJ, O'morain C, Bolling-Sternevald E, Sundin M, Eriksson S, Blum A. In patients with Helicobacter pylori gastritis and functional dyspepsia, a biopsy from the incisura angularis provides useful diagnostic information. Pathol Res Pract. 2006;202:405–413. doi: 10.1016/j.prp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Dursun M, Yilmaz S, Yükselen V, Kilinç N, Canoruç F, Tuzcu A. Evaluation of optimal gastric mucosal biopsy site and number for identification of Helicobacter pylori, gastric atrophy and intestinal metaplasia. Hepatogastroenterology. 2004;51:1732–1735. [PubMed] [Google Scholar]
- 26.Ola SO, Yakubu A, Otegbayo JA, Oluwasola AO, Ogunbiyi JO, Akang EE, Summerton CB. The most appropriate site for endoscopic biopsy for the detection of H. pylori among Nigerians in Ibadan. West Afr J Med. 2006;25:269–272. [PubMed] [Google Scholar]