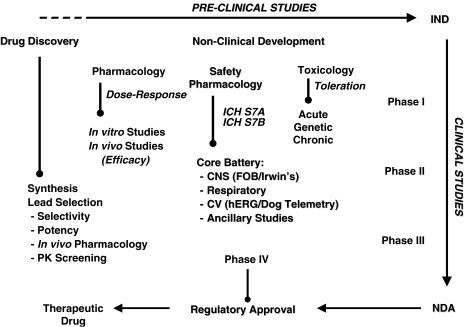
Figure 3.
Schematic depicting the complex interaction of preclinical scientific disciplines and study models used to characterize the safety profile of a new chemical entity. A non-clinical development programme includes data from drug discovery models up through Safety Pharmacology and Toxicology where an investigational new drug application (IND) is filed for a candidate drug. The IND is the means by which a pharmaceutical company obtains regulatory permission (from the FDA) to provide drug to clinical investigators for use in phase I clinical trials. The FDA reviews the IND application for safety to assure that clinical research subjects will not be subjected to unreasonable risk. The candidate drug then proceeds through multiple clinical trials (phase I–III) after which an NDA or new drug appliation is made to regulatory authorities. In this document drug sponsors propose that the FDA approve a new pharmaceutical for sale and marketing. The goals of the NDA are to provide enough information to permit FDA reviewers to establish whether the drug is safe and effective for its proposed indication.