Fig. 1.
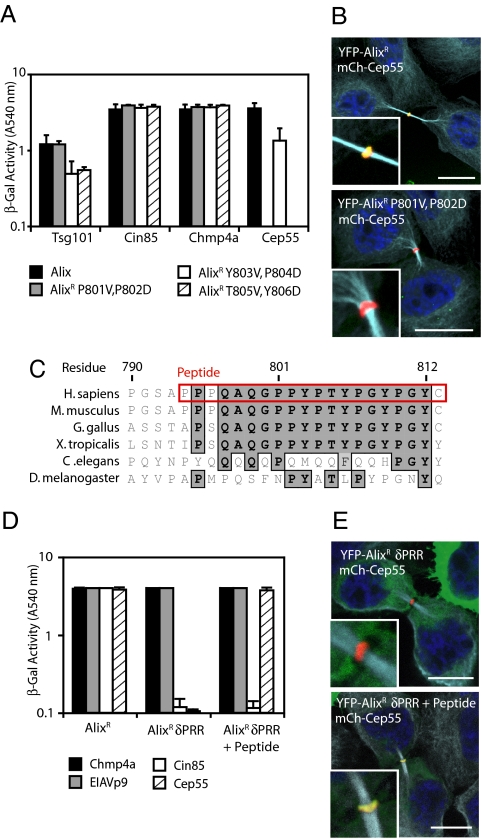
Cep55 recruits Alix to the midbody through binding a conserved peptide within Alix's PRR. (A) Alix mutants were fused to the VP16-activation domain and tested for interaction with the indicated proteins fused to Gal4 DNA-binding domain by yeast two-hybrid assay (n = 3 ± SD). (B) HeLa cells were transfected with plasmids encoding mCh-Cep55 and either YFP-AlixR or YFP-AlixR P801V, P802D, fixed and stained with α-tubulin. (Scale bars: 10 μm.) (C) Sequence alignment of the Cep55-binding region of the Alix-PRR with the Cep55-binding peptide highlighted in red. (D) Alix, AlixR δPRR, and AlixR δPRR plus peptide were fused to the VP16 DNA-activation domain and tested for interactions with the indicated Gal4 DNA-binding domain fusions by yeast two-hybrid assay (n = 3 ± SD). (E) HeLa cells were transfected with plasmids encoding mCh-Cep55 and either YFP-AlixR or YFP-AlixR δPRR and AlixR δPRR plus peptide, fixed, and stained with α-tubulin. (Scale bars: 10 μm.)