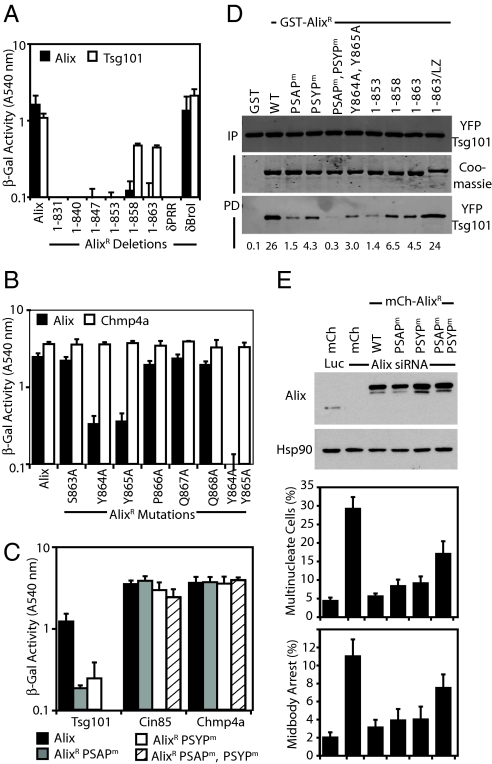
Fig. 4.
Activities in the C terminus of Alix. (A and B) The indicated Alix constructs fused to the VP16-activation domain were tested for interactions with Alix or Tsg101 fused to the Gal4 DNA-binding domain by yeast two-hybrid assay (n = 3 ±SD). (C) The indicated Alix mutants were fused to the VP16-activation domain and tested for interactions with Tsg101, Cin85, and Chmp4a fused to the Gal4 DNA-binding domain by yeast two-hybrid assay (n = 3 ±SD). (D) Coprecipitation assays were performed on lysates of 293T cells cotransfected with plasmids encoding YFP-Tsg101, Myc-Vps28, and the indicated GST-AlixR fusion proteins. Resolved cells lysates (input, IP) and normalized bead-bound fractions (pull down, PD) were analyzed with α-GFP antisera. PD fractions were analyzed by Coomassie staining to visualize GST-AlixR expression, and relative binding was quantified by infrared imaging. (E) HeLa cells stably transduced with retroviral vectors encoding mCh or the mCh-AlixR fusions were treated with siRNA targeting Luciferase or Alix. Cells were fixed, stained with α-tubulin, and scored for midbody arrest and multinucleation (n = 3 ±SD). Resolved cell lysates were analyzed with α-Alix and α-Hsp90 antibodies.