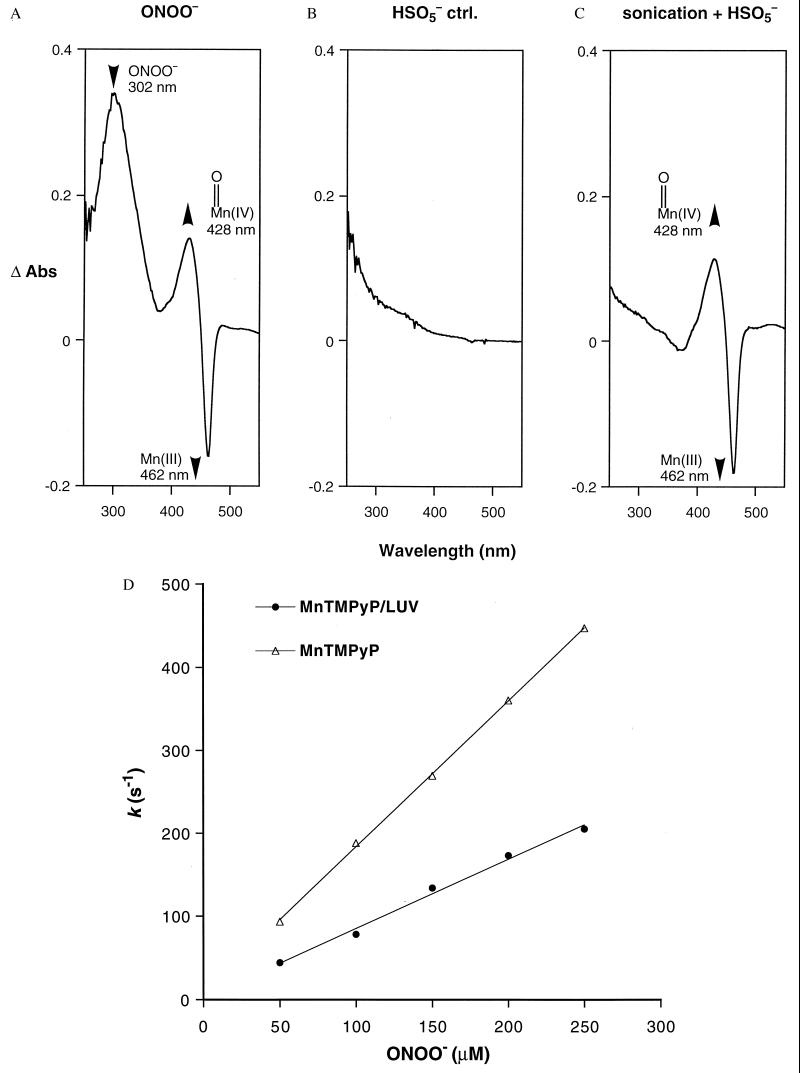
Figure 3.
ONOO− is shown to cross membranes freely. Difference spectra of the UV-vis absorbances before and after addition of oxidants to LUV—encapsulated Mn(III)TMPyP. (A) ONOO− (1 mM) addition resulted in an instantaneous oxidation of Mn(III) (462 nm) to the oxoMn species (428 nm), indicating that ONOO− diffused rapidly across the lipid bilayer. Rapid oxidation was seen between pH 7.4 and 8.8, suggesting that the ONOO− anion as well as HOONO can cross membranes (pKa ONOO− 6.8). Similar results were obtained with OCl−. (B) Addition of HSO5− (1 mM) to the Mn(III)TMPyP/LUV resulted in no oxidation, verifying that the LUV prevented intermixing of the entrapped porphyrin and HSO5− in this construct. (C) Oxidation by HSO5− was observed only after the LUV had been sonicated to relocate Mn(III)TMPyP into the bulk solution. (D) Comparison of the pseudo first-order rates for the solution reaction compared with the reaction in LUV. All of the experiments were conducted using rapid mixing stopped-flow methods as described in Materials and Methods. Reaction rates obtained from the slope of the pseudo first-order plots were: kintrinsic = 1.8 × 106 M−1⋅s−1 (R = 0.999); and kobsd = 0.8 × 106 M−1⋅s−1 (R = 0.996).