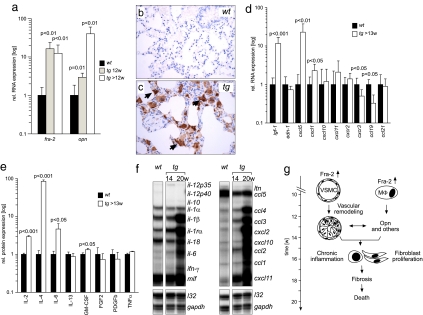
Fig. 4.
Molecular factors involved in pulmonary fibrosis in fra-2tg mice. (a) Expression of fra-2 and osteopontin (opn) was analyzed by quantitative RT-PCR at the indicated time points (n ≥ 5). (b and c) Immunohistochemistry reveals that osteopontin, absent from the wild type (b), was predominantly expressed by alveolar macrophages (c, arrows) in fra-2tg lungs (17 weeks of age, n = 3). (d) Expression of fibrosis-related factors in fra-2tg lungs was analyzed by quantitative RT-PCR (n ≥ 5). igf-1, insulin like growth factor 1; edn-1, endothelin 1. (e) ELISA demonstrating increased cytokine protein expression of IL-2, IL-4, IL-6, and GM-CSF in lungs of fra-2tg mice (n ≥ 4). (f) Pulmonary expression of cytokines/chemokines in fra-2tg (tg) mice that appeared healthy (14 weeks) or obviously sick (20 weeks). l32 and gapdh were used as loading controls. (g) Proposed pathway leading to pulmonary fibrosis in fra-2tg mice. Increased activity of Fra-2 in VSMCs, pulmonary epithelium (not shown), and alveolar macrophages (MΦ) induces aberrant vascular remodeling at ≈12 weeks of age, presumably by regulating osteopontin expression. These alterations lead to chronic inflammation, tissue damage, expression of profibrogenic factors such as IL-4, subsequent activation and proliferation of matrix-producing cells, fibrosis, and premature mortality.