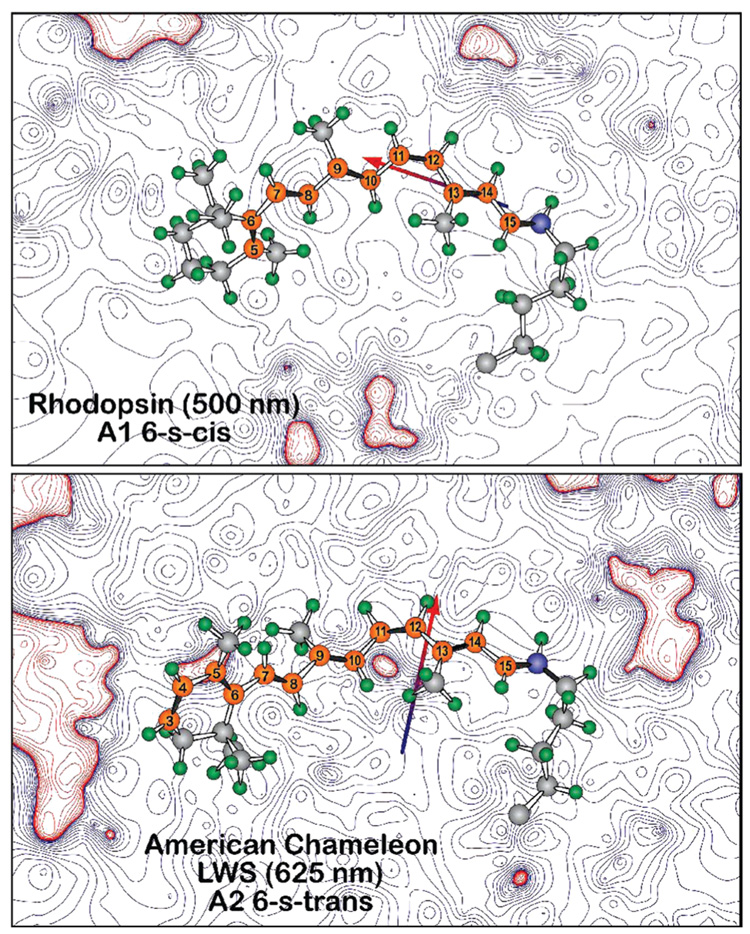
FIGURE 5.
Electrostatic contours in the plane of the chromophores of bovine rhodopsin (1U19) and a 6-s-trans homology model of American chameleon based on 1U19. The contours are based on Mulliken charges from a single SCF PM3 Mozyme calculation on a Charmm-based structure following 2 ns molecular dynamics. The contours are associated with the protein residues, ignoring the chromophore charges. Note that, in both cases, the chromophore is bathed in an electrostatic field that is predominantly negative (blue contours). However, the dipole moment of the binding site is nearly orthogonal between the two proteins, with the rhodopsin binding site favoring a 6-s-cis conformer (top) and the American chameleon binding site (bottom) favoring a 6-s-trans conformer.