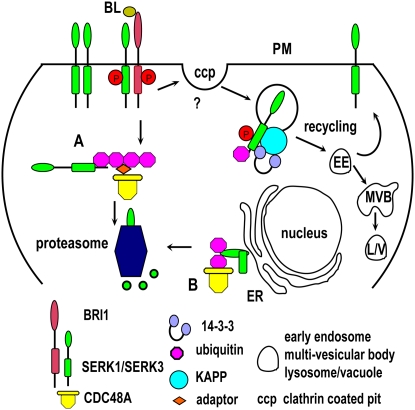
Figure 2.
Hypothetical degradation pathways of the SERK1 receptor after activation. Upon binding of the BR brassinolide, BRI1, and SERK1 (and/or SERK3), receptors dimerize and transphosphorylate. After activation, monoubiquitination is a signal for internalization (via clathrin-coated pits?), acquiring the phosphatase KAPP, to give rise to early endosomes. From these endosomes, the receptors can recycle to the PM or are targeted to MVBs and subsequently to lysosomes or vacuoles to be degraded. A, Polyubiquitination after activation of the receptors is a signal for degradation in the proteasome, acquiring adaptor proteins and CDC48A. B, Misfolded SERK1 receptors are pulled out of the ER lumen, polyubiquitinated, and targeted to the proteasome, acquiring CDC48A again.