Figure 1.
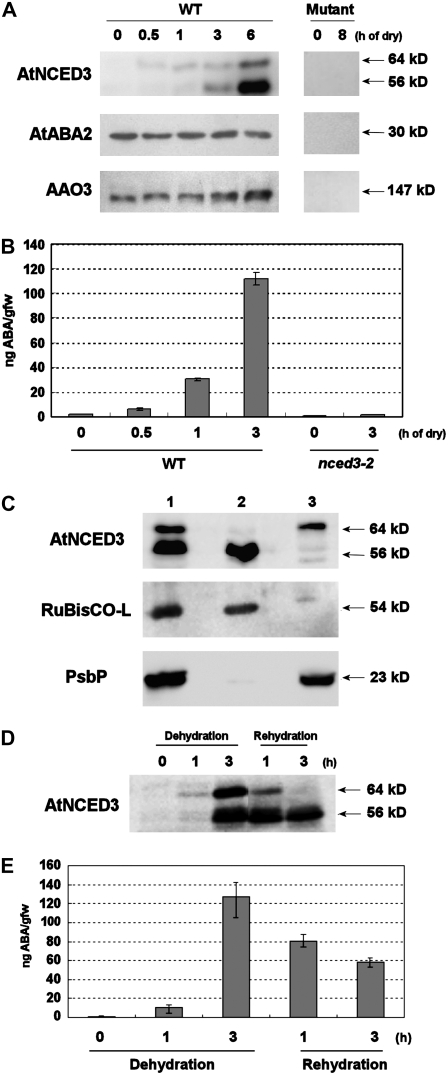
Specific recognition of the ABA biosynthesis enzymes by polyclonal antibodies. A, Western-blot analysis of protein extracts from turgid and dehydrated plants. Two-week-old wild-type and mutant plants (AtNCED3, nced3-1; AtABA2, aba2-2; and AAO3, aao3-4) were subjected to dehydration as indicated at top. Forty micrograms of total protein was loaded in each lane and immunoreacted with antibodies against AtNCED3, AtABA2, and AAO3. The molecular masses of ABA biosynthetic enzymes are indicated along the right side. B, ABA measurement of wild-type and nced3-2 mutant plants after dry stress treatment. Two-week-old wild-type and nced3-2 mutant plants were subjected to dehydration treatment during 0 to 3 h (as indicated at bottom). ABA levels normalized by initial fresh weigh prior to dehydration are shown. Error bars indicate se (n = 3). C, Western-blot analysis of chloroplast proteins from dehydrated plants. Intact chloroplasts were isolated from wilted Arabidopsis rosette leaves using a Percoll gradient. Proteins from the intact chloroplast (lane 1), stroma plus envelope membrane (lane 2), and thylakoid fraction (lane 3) were analyzed by western blotting with anti-AtNCED3, anti-spinach Rubisco-L, and anti-PsbP antibodies, respectively. The molecular masses of AtNCED3, Rubisco-L, and PsbP are indicated along the right side. D, Western-blot analysis of AtNCED3 upon rehydration. Analysis was performed with protein samples extracted from 2-week-old plants subjected to 0 to 3 h of dehydration and 1 and 3 h of subsequent rehydration. AtNCED3 was detected using anti-AtNCED3 antibodies. Molecular masses are indicated along the right side. E, Changing endogenous ABA levels during dehydration and rehydration. Two-week-old wild-type plants were subjected to dehydration for 0, 1, and 3 h. For rehydration treatment, plants were rehydrated for 1 or 3 h after 3 h of dehydration (as indicated at bottom). ABA levels normalized by initial fresh weigh prior to dehydration are shown. Error bars indicate se (n = 3).