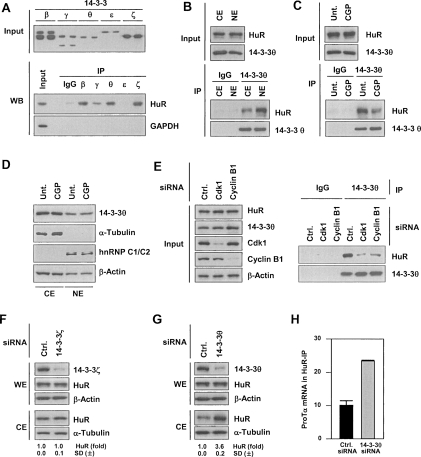
Figure 4.
Phosphorylated HuR binds 14–3–3θ and localizes in the nucleus. (A, top) Western blot analysis of the indicated 14–3–3 isoforms (paired samples). (Bottom) Various 14–3–3 isoforms were immunoprecipitated using specific antibodies, and the presence of HuR and negative control GAPDH in the IP materials was tested by Western blot analysis. (B) CE and NE were prepared from HeLa cells, used for IP with control rabbit IgG or anti-14–3–3θ antibodies, and HuR and 14–3–3θ in the IP samples was detected by Western blot analysis. (C) Following treatment with CGP (2 μM, 2 h), WE were prepared and assayed by IP using control rabbit IgG or anti-14–3–3θ antibodies; bound HuR and 14–3–3θ were assessed by Western blotting. (D) After CGP treatment (2 μM, 2 h), CE and NE were prepared and the levels of 14–3–3θ, the cytosolic marker α-Tubulin, the nuclear marker hnRNP C1/C2, and the loading control β-Actin were analyzed by Western blotting. (E) Forty-eight hours after transfection of cells with the siRNAs shown, WE were prepared and used for IP in the presence of rabbit IgG or anti-14–3–3θ antibodies. (Left) The levels of HuR, Cdk1, cyclin B1, and loading control β-Actin in the Input material were tested by Western blotting. (Right) The presence of HuR and 14–3–3θ in the IP samples was assessed by Western blotting. (F,G) Cells were transfected with either control (Ctrl.) siRNA or with an siRNA targeting 14–3–3ζ or 14–3–3θ. Forty-eight hours after transfection, CE and WE were prepared and the levels of HuR and 14–3–3ζ (F) and 14–3–3θ (G) were detected by Western blotting; β-Actin and α-Tubulin were used as loading controls; ±SD of HuR signals from at least three experiments are indicated. (H) In cells transfected with 14–3–3θ siRNA, ProTα mRNA levels in HuR IP were determined by RT–qPCR (Materials and Methods); graph depicts the means and SD from three independent experiments.