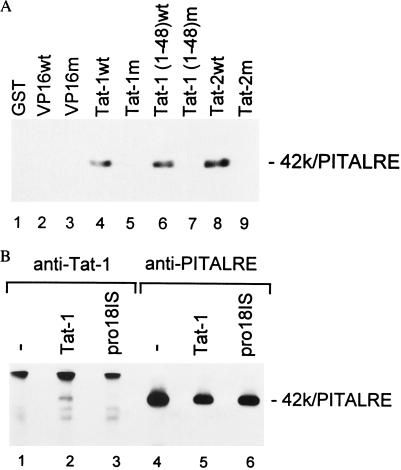
Figure 5.
Specific association of PITALRE with Tat proteins in vitro and in vivo. (A) GST-fusion protein-binding assays. Binding assays were performed as described in Materials and Methods with a HeLa nuclear extract and GST protein (lane 1) or GST fusions to the VP16 wild-type (wt) activation domain (lane 2), a mutant VP16 activation domain (F442S+F475A) (9) (lane 3), Tat-1 wt 86R protein (lane 4), mutant Tat-1 pro18IS 86R protein (lane 5), wt Tat-1 48R activation domain (lane 6), mutant Tat-1 48R pro18IS activation domain (lane 7), wild-type Tat-2 99R protein, and mutant Tat-2 99R C59A protein. The products were separated on a 9% SDS/polyacrylamide gel, and the presence of PITALRE was evaluated by an immunoblot using anti-PITALRE antibodies. Coomassie blue staining of a gel run in parallel with that shown demonstrated that all GST-fusion proteins were present at equivalent levels in pull-downs. (B) Immunoprecipitation immunoblots. HeLa cells were mock-infected or infected with a recombinant adenovirus expressing the wild-type Tat-1 72R protein or the Tat-1 transactivation-defective pro18IS 72R mutant. Extracts were prepared at 48 hr postinfection, and immunoprecipitations were performed with an antiserum against Tat-1 or PITALRE as indicated. Immunoprecipitates were separated on an 8% SDS/polyacrylamide gel, and the presence of PITALRE was evaluated by an immunoblot with antibodies against PITALRE.