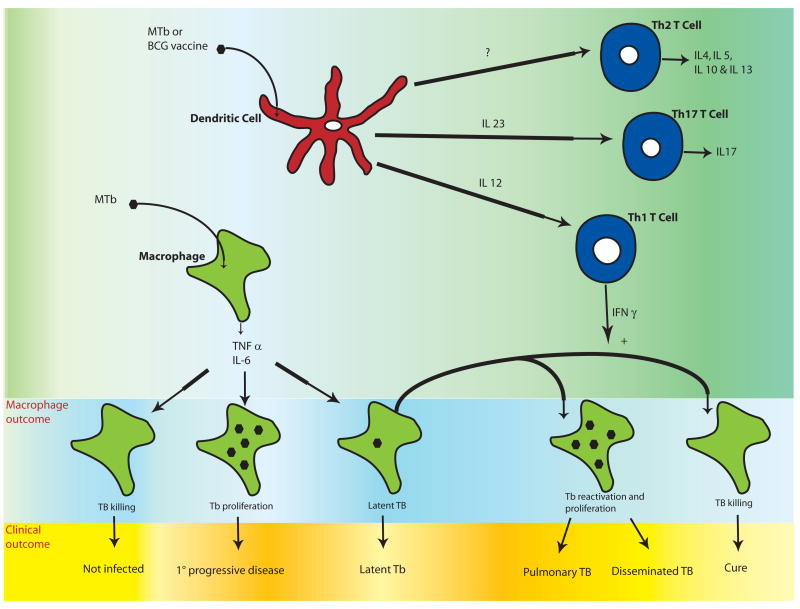
Figure 1. Clinical and cellular outcomes of Mycobacterium tuberculosis (MTb) and the role of the adaptive immune system.
Initial MTb infection of the macrophage results in production of pro-inflammatory cytokines such as IL-6, IL-1β, IL-12, and TNF-α. Unknown factors in host defense lead to a spectrum of initial clinical outcomes including resistance to infection, primary progressive disease, and latency. In latent infection, there is a balance of mycobacterial proliferation and host defense and MTb is contained in granulomas with no clinical symptoms. Ten percent of those with latent MTb infection will eventually develop clinically active disease that manifests as localized pulmonary infection (in 80% of individuals) or disseminated disease. These diverse clinical outcomes may be regulated by variation in the innate immune response of macrophages and/or dendritic cells. T cell responses are shaped by interactions with dendritic cells, which depend on the innate immune response to MTb or BCG. Th1 T cells produce IFN-γ and promote mycobacterial killing of TB-infected macrophages. Th17 T cells secrete IL-17 and may be important for protective vaccine-induced responses. The role of Th2 T cells in host defense to MTB infection is less clear. The combination of innate and adaptive responses influences the macrophage response to TB infection as well as the clinical outcome. Immunomodulatory therapy directed at innate immune receptors could potentially alter clinical outcomes by affecting several steps in disease pathogenesis outlined in this figure.