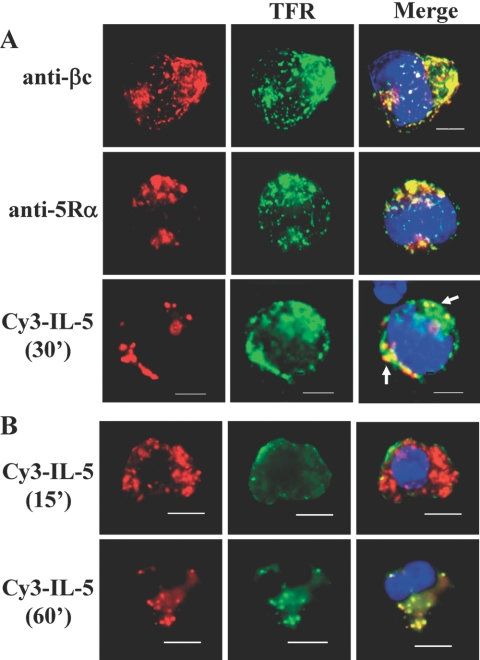
Fig. 1.
IL-5Rs colocalize with transferrin-positive vesicles (clathrin-containing). (A) Cytokine-starved TF1 cells were serum starved and incubated with cold transferrin-488 (10 μg/ml) and cold unlabeled IL-5 (10 ng/ml) for 30 min as described in Materials and Methods. For dual uptake assays, 250 ng/ml Cy3-IL-5 and 10 μg/ml Tfr-488 were added together. Cells were treated with acid wash, fixed, stained with anti-βc (upper panels) or anti-IL-5Rα (middle panels), and mounted with ProLong Gold reagent, which contains a DAPI stain (blue) for nuclear detection. Images were collected and analyzed by deconvolution microscopy. Note the colocalization (merge) of βc (upper right panel), 5Rα (middle right panel), and Cy3-IL-5 (lower right) with Tfr-488. Percentage of cells counted showing βc/Tfr co-localization was 77%; 67% for 5Rα/Tfr; and 35% for Cy3-IL-5/Tfr. Raw cell counts are listed in Table 1. Scale bar = 5 μm. (B) Cy3-IL-5 and Tfr-488 (clathrin-positive) colocalize in human eosinophils. After isolation from leukopaks, freshly isolated eosinophils were “synchronized” overnight in RPMI medium containing 10% FBS (no IL-5). Dual uptake assays with Cy3-IL-5 (250 ng/ml) (red) and Tfr-488 (green) were performed exactly as described for TF1 cells, and colocalization was visualized by deconvolution microscopy at 15 and 60 min after initiation of internalization at 37°C. Nuclei are visualized with DAPI stain. These results are representative of 4 independent experiments (n=4) with a minimum of 80-100 cells analyzed.