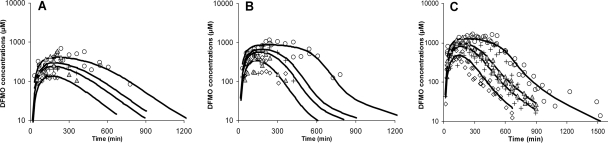
FIG. 4.
Experimental values and mean population predicted values (black lines) for l-eflornithine (A), d-eflornithine (B), and racemic eflornithine (C) after oral administration of racemic eflornithine hydrochloride to Sprague-Dawley rats at doses of 750 (diamonds), 1,500 (crosses), 2,000 (triangles), and 3,000 (circles) mg/kg of body weight. l-, d-, and racemic eflornithine data were fitted simultaneously to obtain absorption kinetic parameters for each eflornithine enantiomer, using a two-compartment disposition model with parameters fixed from the intravenous data. The absorption data were described with a transit model transferring drug to an absorption compartment, from which the systemic absorption rate was characterized by a Michaelis-Menten-type function.