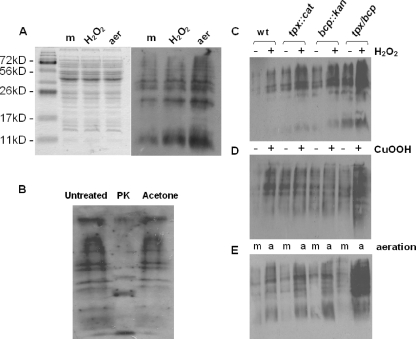
FIG. 4.
Effect of tpx and bcp mutations on protein carbonyl content. (A) Relative levels of protein carbonylation in wild-type cells grown microaerobically to early stationary phase with no further treatment (m), exposed to 1 mM hydrogen peroxide for a further 2 h (H2O2), or incubated with vigorous shaking at high aeration (21% [vol/vol] oxygen) for 2 h (aer). (Left panel) Coomassie blue-stained 12% (wt/vol) SDS-polyacrylamide gel containing equivalent amounts of wild-type CE protein. (Right panel) Corresponding immunoblot with anti-DNP antibodies. (B) Control immunoblot assay to confirm that the detected bands are carbonylated proteins. Aliquots of a CE from aerated cells were either left untreated, incubated with 200 μg ml−1 proteinase K (PK) at 37°C for 16 h, or treated with 2 volumes of −20°C acetone to precipitate protein and remove lipid. Samples were then derivatized as described in Materials and Methods, and equal loadings were applied to a 12% (wt/vol) SDS-polyacrylamide gel and immunoblotted with anti-DNP antibodies. (C to E) Levels of protein carbonylation detected on anti-DNP immunoblots in wild-type (wt) and mutant strains in the presence (+) and absence (−) of 1 mM hydrogen peroxide (C) or 0.1 mM cumene hydroperoxide (D) or after growth at high aeration (E), under 21% (vol/vol) oxygen (a) versus microaerobically (m).