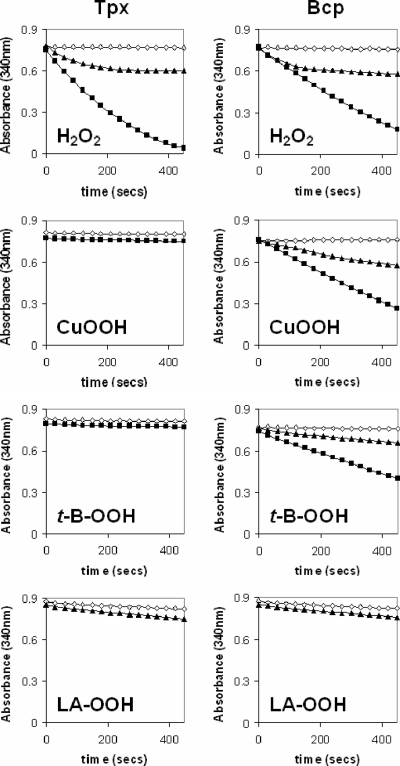
FIG. 6.
Peroxidase activities of purified enzymes using the thioredoxin system as reductant. Each panel is an absorbance time course at 340 nm (due to NADPH oxidation) in the absence (open diamonds) or the presence (closed symbols) of the peroxide substrate shown, using purified Tpx (left column) or Bcp (right column). The reaction mixture contained 50 mM HEPES-NaOH (pH 7.0), 20 μM NADPH, 20 μg E. coli Trx, 6.25 μg E. coli TrxR, 1 μM pure thiol peroxidase enzyme (Tpx or Bcp), and either 20 μM (closed triangles) or 100 μM (closed squares) peroxide substrate. Reactions were carried out in a total volume of 1 ml at 37°C. The reaction was started by the addition of NADPH. LA, linoleic acid.