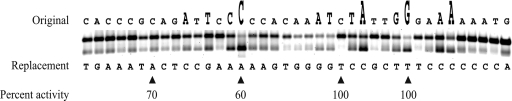
FIG. 1.
Mutation-based characterization of the ExpR binding site upstream of sinI. Purified His6-ExpR was mixed with 10 μM oxo-C14-HL and Cy3-labeled UpsinI fragments, each carrying a single point mutation, and the effect of the mutation was determined using gel shift assays. Each shift is a representation of the effect caused by a single modifying nucleotide on ExpR-DNA binding strength. A weak shift indicates a significant disruptive effect caused by the modification, and a strong shift indicates a moderate or negligible effect. The letter above each shift indicates the original nucleotide, and the letter below each shift indicates the modifying nucleotide. The nucleotides very important for ExpR binding are indicated by a large increase in the size of the letter above the shift; nucleotides of lesser importance are indicated by an intermediate increase in size. The resulting shift using the native Cy3-labeled UpsinI fragment (not shown) showed no observable difference from the first five shifts on the left side of the figure. The gel shift experiment was repeated three times. Four UpsinI fragments, each with a single point mutation (indicated by the arrows below), were cloned into the promoter probe vector, in addition to the native UpsinI fragment. Percent activity indicates the β-galactosidase activity that was measured from the mutated fragment in comparison to that from the native fragment.