Figure 4. Structural comparison of PP5 to other members of the PPP.
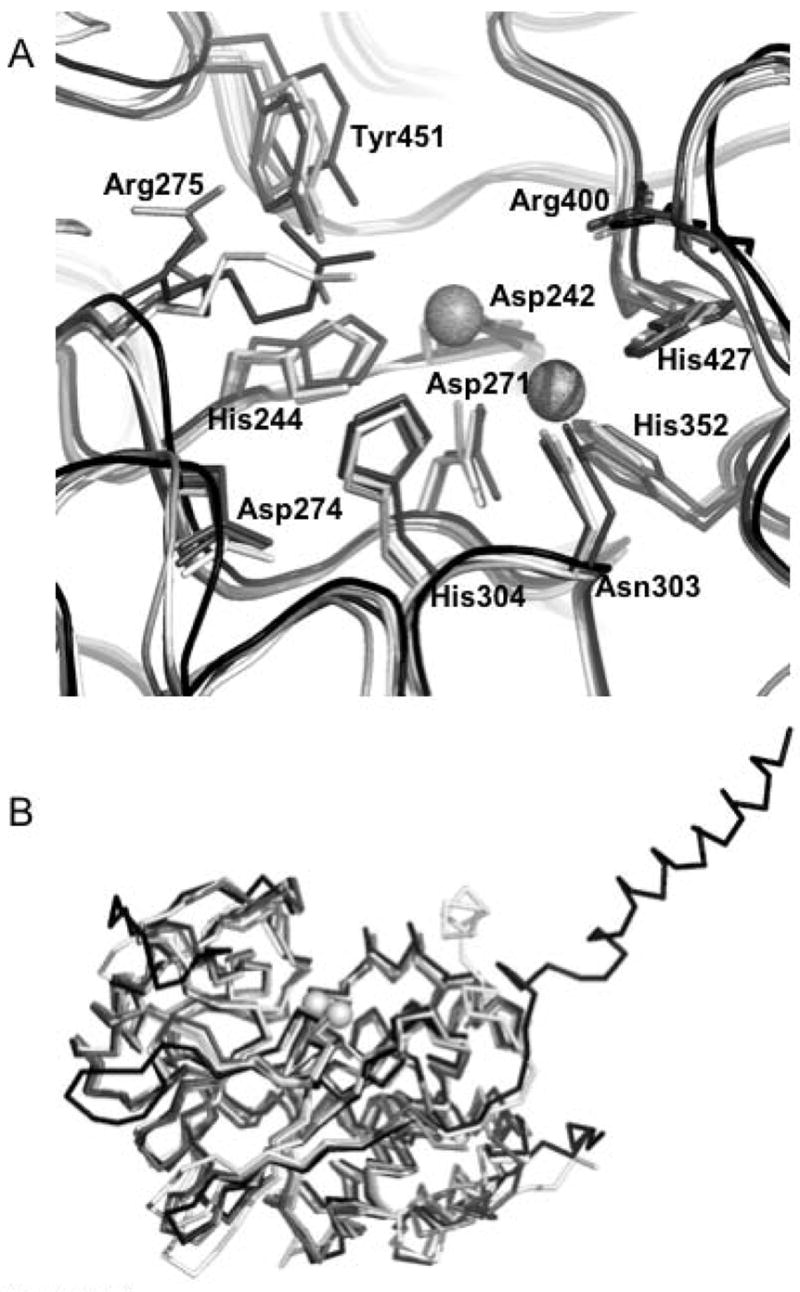
family. A. Superposition of the active sites of (1S95:white), PP2A (2IE4:light gray), PP1 (1JK7:dark gray), and PP2B (1TCO:black) showing the positions and conformations of 11 absolutely conserved residues. The active site metal positions are derived from 1JK7. PP5 numbering of residues is observed. B. Superposition (shown as Cα traces) of the catalytic domains of PP5 (1S95:white), PP2A (2IE4:light gray), PP1 (1JK7:dark gray), and PP2B (1TCO:black). The active site metal positions are derived from 1JK7. These structural alignments show the remarkable similarity of the overall fold in these four representatives of the PPP family. This similarity is particularly evident in and around the catalytic center where the conserved residues are found in essentially identical conformations (an exception being Arg275 which takes on different conformations depending upon which ligand is bound in the active site). Structural alignments were done with STRAP (http://www.charite.de/bioinf/strap/) and the figure was prepared with PyMOL (http://www.pymol.org).
