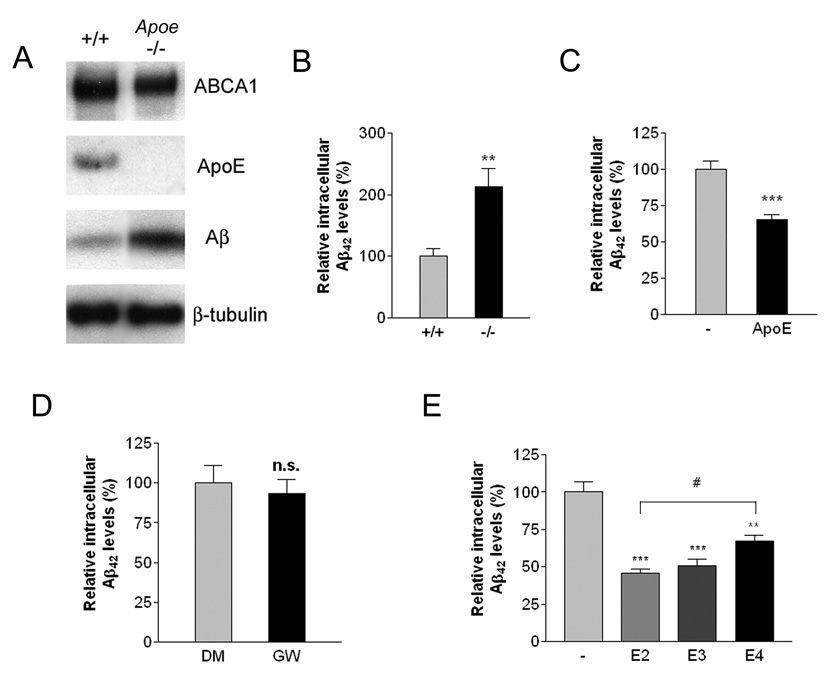
Figure 4. ApoE is essential for efficient degradation of Aβ.
(A) Loss of ApoE resulted in intracellular accumulation of Aβ. Primary microglia from wild type or Apoe −/− mice were treated with 2 µg/ml Aβ42 for 24 hours. The lysates were subjected to SDS-PAGE and Western blotted for ABCA1, ApoE, Aβ and β-tubulin as a loading control. Blots shown are representative of three independent experiments. (B) Intracellular Aβ levels were quantified using ELISA for Aβ42 and normalized to total protein. The data represent the outcome of 5 independent experiments (**P<0.01). (C) Exogenous ApoE rescued the Aβ degrading deficiency in Apoe −/− microglia. Primary microglia from Apoe −/− mice were incubated with 2 µg/ml Aβ42 in the absence or presence of 1 µg/ml ApoE for an additional 24 hours. Intracellular Aβ42 levels were quantified using ELISA and the data normalized to total protein (***P<0.001). (D) ApoE is required for LXR-mediated effect on Aβ degradation. Primary microglia from Apoe −/− mice were pretreated with DMSO or 1 µM GW3965 for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 for an additional 24 hours. Intracellular Aβ42 levels were quantified using ELISA and data normalized to total protein. (E) Native ApoE particles enhanced Aβ degradation in an isoform-dependent manner. Primary microglia from ApoE −/− mice were treated with 2 µg/ml Aβ42 in the absence or the presence of 200 ng/ml purified ApoE-containing native HDL particles isolated from immortalized astrocytes expressing the human ApoE isoforms for 24 hours. The levels of remaining intracellular Aβ were quantified using ELISA for Aβ42. The data represent the outcome of 4 independent experiments (**P<0.01; ***P<0.001; #P<0.05).