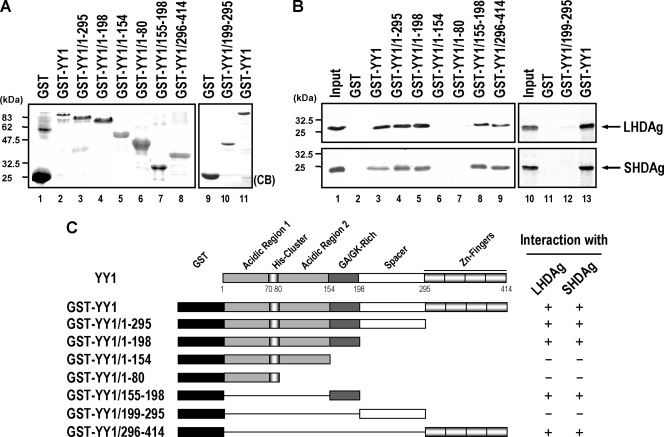
FIG. 3.
Mapping of the HDAg-interacting regions in YY1. (A) Partially purified GST-YY1 and its various truncation mutants (5 μg each) were separated by SDS-PAGE and detected by Coomassie brilliant blue (CB) staining. (B) In vitro binding assay of GST-YY1 variants and in vitro translated 35S-labeled HDAgs. Glutathione-Sepharose beads (20 μl) bound with GST (lanes 2 and 11), GST-YY1 (lanes 3 and 13), or GST-YY1 variants (lanes 4 to 9 and 12) (5 μg each) were incubated with in vitro translated 35S-labeled LHDAg or SHDAg (5 μl), and proteins bound on the beads were eluted and analyzed by SDS-PAGE followed by autoradiography. Input, in vitro translated 35S-labeled LHDAg or SHDAg (5 μl for lane 1 and 1 μl for lane 10). (C) A schematic diagram showing the binding regions of HDAgs within YY1. The numbers represent the positions of the amino acid residues of YY1. The binding ability of LHDAg or SHDAg with GST-YY1 or its truncation variants is indicated by a plus or a minus sign.