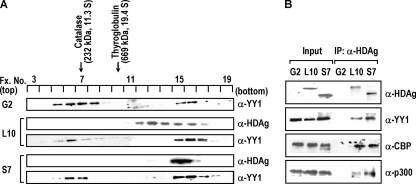
FIG. 4.
YY1 forms a large complex with HDAgs in vivo. (A) The nuclear extracts (500 μg) of the HDAg-producing (L10 or S7) and the parental HepG2 (G2) cells were isolated and subjected to sucrose gradient centrifugation analysis. Aliquots of each fraction were analyzed by immunoblotting using antibodies against YY1 or HDAg. Protein standards, catalase (232 kDa) and thyroglobulin (669 kDa), were run in parallel experiments, and their sedimented positions are indicated. (B) Complex formation between HDAgs, YY1, CBP, and p300. The sucrose gradient fractions 15 to 17 obtained from nuclear extracts of HepG2 (G2), L10, or S7 cells as described in panel A were pooled together and immunoprecipitated (IP) with human anti-HDAg antiserum. The immunoprecipitates were analyzed by SDS-PAGE followed by immunoblotting with antibodies against HDAg, YY1, CBP, and p300. Input, nuclear extracts (50 μg) of G2, L10, or S7 as loading controls; α, anti.