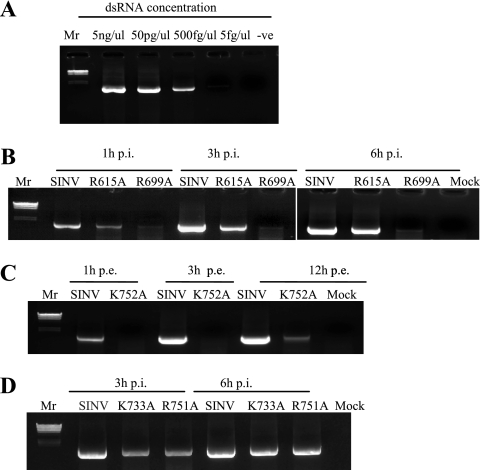
FIG. 5.
Detection of minus-strand RNA by RT-PCR. (A) dsRNA was prepared by hybridizing plus-sense and minus-sense in vitro transcripts representing the 5′ terminal 3.0-kb region of the SINV genome. The dsRNA was serially diluted in water (the concentration of RNA is shown at the top) and used in a minus-strand-specific RT-PCR assay to amplify a 1.6-kb fragment; -ve, one-step RT-PCR negative control. (B, C, and D) Minus-strand-specific RT-PCR for cytoplasmic RNA derived from cells infected with wild-type (SINV) or mutant virus at an MOI of 10 (B and C) or transfected with 10 μg of RNA carrying the mutation (D). The PCR amplification was for 30 cycles except in the case of K733A and R751A (C), where the amplification was 15 cycles long for the 6-h time point. Lambda phage DNA digested with HindIII was used for size standards (Mr). Cytoplasmic RNA from uninfected cells was used as a control (Mock). p.i. postinfection; p.e., postelectroporation.