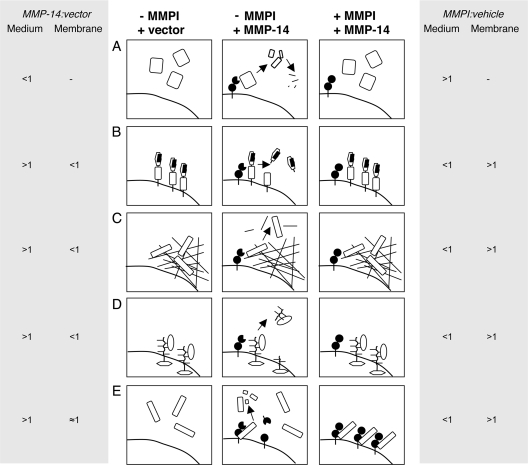
FIG. 1.
Hypothesis: the MMPI attenuates shedding and release of cleaved proteins into the conditioned medium. Without MMP-14 (left panels, −MMPI + vector), no MMP-14-mediated processing occurs. With MMP-14 but in the absence of the MMPI (center panels, −MMPI +MMP-14), active MMP-14 on the cell membrane (A) processes secreted proteins, which may result in further cleavages and clearance by MMPs or other proteases; (B) sheds membrane-associated or integral membrane proteins or their binding partners from the cell surface; (C) processes or releases proteins from extracellular and pericellular matrix; or (D) sheds directly or indirectly mobilizes secreted proteins from cell binding sites, e.g., by processing proteoglycans or integrins. These events will be blocked by a broad-spectrum MMPI (right panels, +MMPI +MMP-14). In the presence of an MMPI, soluble substrates increase in the conditioned medium (A). Whether the ratio changes or not will depend upon the rate of clearance of any fragments which will still be quantified as labeled tryptic peptides. Previously shed cell- or matrix-associated proteins decrease in the conditioned medium (B, C, and D), which coincides with their increase in the membrane or matrix. A similar response might be caused by MMPI-induced dominant-negative effects (E). Autodegradation of MMP-14 (center panel) is prevented by the MMPI, leading to an accumulation of mature MMP-14 at the cell surface (right panel). These inhibited MMP-14 molecules could act as “substrate traps,” binding substrates (and other interacting molecules) at exosites without cleavage and release. Hence, shed and soluble proteins would be titrated from the conditioned medium and sequestered at the cell surface. The predicted ICAT ratios for cells transfected with MMP-14 compared with empty vector (MMP-14/vector) and cells transfected with MMP-14 treated with inhibitor drug or vehicle (MMPI/vehicle) are shown adjacent to each panel for proteins in the conditioned medium (Medium) or cell membrane fractions (Membrane).