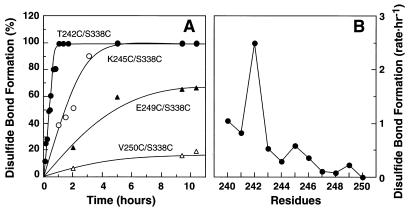
Figure 2.
Rates of disulfide bond formation in double Cys mutants S240C/S338C to V250C/S338C. All the mutant preparations after elution from the Ab 1D4-Sepharose column, were shifted to pH 7.5 as in “Materials and Methods.” The time course of disulfide bond formation was then monitored by measuring the decrease in sulfhydryl groups by reaction with PDS (Materials and Methods). (A) The extent of disulfide bond formation in mutants: T242C/S338C, K245C/S338C, E249C/S338C, and V250C/S338C as a function of time. (B) A plot of the rates of disulfide bond formation in the mutants S240C/S338C– V250C/S338C. The rate is represented by 1/t1/2 (h –1), where t1/2 is the time (h) required for reaching 50% disulfide bond formation. t1/2 for each mutant was derived from the data in A.