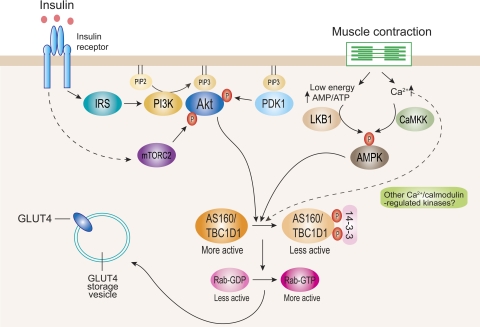
Fig. 1.
Currently proposed signaling pathways for insulin- and contraction-stimulated glucose transporter 4 (GLUT4) translocation in muscle. Insulin stimulates Akt through 2 distinct upstream mediators, phosphoinositide-dependent protein kinase-1 (PDK1) and mammalian target of rapamycin complex-2 (mTORC2). Activated Akt phosphorylates AS160 and TBC1D1 mainly at Thr642 and Thr596, respectively. This enhances 14-3-3 binding to these proteins, which is proposed to inhibit Rab-GTPase-activating protein (GAP) activity toward particular Rab isoform(s). Inhibition of GAP promotes conversion of less active GDP-loaded Rab to more active GTP-loaded Rab. The more active GTP-loaded Rab then allows GLUT4 storage vesicles to move to and fuse with the plasma membrane. Contraction through both energy depletion (i.e., an elevated AMP/ATP ratio) and elevated intracellular [Ca2+] leads to activation of AMP-activated protein kinase (AMPK) via LKB1 and Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), respectively (and possibly other Ca2+-regulated protein kinases). These regulators lead to AS160 and TBC1D1 phosphorylation at multiple phosphorylation sites. This is thought to regulate function of these proteins and GLUT4 trafficking by largely uncharacterized mechanism(s). IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase.